Abstract
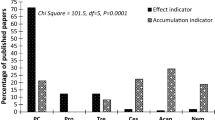
The aquatic environment represents the final repository for many human-generated pollutants associated with anthropogenic activities. The quality of natural freshwater systems is easily disrupted by the introduction of pollutants from urban, industrial and agricultural processes. To assess the extent of chemical perturbation and associated environmental degradation, physico-chemical parameters have been monitored in conjunction with biota in numerous biological monitoring protocols. Most studies incorporating organisms into such approaches have focussed on fish and macroinvertebrates. More recently, interest in the ecology of parasites in relation to environmental monitoring has indicated that these organisms are sensitive towards the quality of the macroenvironment. Variable responses towards exposure to pollution have been identified at the population and component community level of a number of parasites. Furthermore, such responses have been found to differ with the type of pollutant and the lifestyle of the parasite. Generally, endoparasite infection levels have been shown to become elevated in relation to poorer water quality conditions, while ectoparasites are more sensitive, and exposure to contaminated environments resulted in a decline in ectoparasite infections. Furthermore, endoparasites have been found to be suitable accumulation indicators for monitoring levels of several trace elements and metals in the environment. The ability of these organisms to accumulate metals has further been observed to be of benefit to the host, resulting in decreased somatic metal levels in infected hosts. These trends have similarly been found for host–parasite models in African freshwater environments, but such analyses are comparatively sparse compared to other countries. Recently, studies on diplozoids from two freshwater systems have indicated that exposure to poorer water quality resulted in decreased infections. In the Vaal River, the poor water quality resulted in the extinction of the parasite from a site below the Vaal River Barrage. Laboratory exposures have further indicated that oncomiracidia of Paradiplozoon ichthyoxanthon are sensitive to exposure to dissolved aluminium. Overall, parasites from African freshwater and marine ecosystems have merit as effect and accumulation indicators; however, more research is required to detail the effects of exposure on sensitive biological processes within these organisms.
Similar content being viewed by others
References
Abdel-Gaber R, Abdel-Ghaffar F, Bashtar A-R, Morsy K, Saleh R (2016) Interactions between the intestinal cestode Polyonchobothrium clarias (Pseudophyllidea: Ptychobothriidae) from the African sharptooth catfish Clarias gariepinus and heavy metal pollutants in an aquatic environment in Egypt. J Helminthol 90:742–752. doi:10.1017/S0022149X15001054
Abdel-Ghaffar F, Abdel-Gaber R, Bashtar A-R, Morsy K, Mehlhorn H, Al Quraishy S, Saleh R (2015) Hysterothylacium aduncum (Nematoda, Anisakidae) with a new host record from the common sole Solea solea (Soleidae) and its role as a biological indicator of pollution. Parasitol Res 114:513–522. doi:10.1007/s00436-014-4213-1
Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P (2006) Seasonality and the dynamics of infectious diseases. Ecol Lett 9:467–484. doi:10.1111/j.1461-0248.2005.00879.x
Anderson TK, Sukhdeo MVK (2010) Abiotic versus biotic hierarchies in the assembly of parasite populations. Parasitology 137:743–754. doi:10.1017/S0031182009991430
Austin A, Avenant-Oldewage A (2009) Ecological parameters of Lamproglena hoi (Copepoda: Lernaeidae) infection on the Bushveld smallscale yellowfish, Labeobarbus polylepis (Boulenger, 1907). Onderstepoort J Vet Res 76:227–234. doi:10.4102/ojvr.v76i2.47
Avenant-Oldewage A, Marx HM (2000) Bioaccumulation of chromium, copper and iron in the organs and tissues of Clarias gariepinus in the Olifants River, Kruger National Park. Water SA 26:569–582
Bagge AM, Valtonen ET (1996) Experimental study on the influence of paper and pulp mill effluent on the gill parasite communities of roach (Rutilus rutilus). Parasitology 112:499–508. doi:10.1017/S0031182000076964
Baker DE, Cone DK (2000) Occurrence of Ergasilus celestis (Copepoda) and Pseudodactylogyrus anguillae (Monogenea) among wild eels (Anguilla anguilla) in relation to stream flow, pH and temperature and recommendations for controlling their transmission among captive eels. Aquaculture 187:261–274
Baker N, Maina J, Greenfield R (2016) Zinc and cadmium concentrations in the house sparrow (Passer domesticus), Thohoyandou, Limpopo, South Africa. In: 7th International Toxicology Symposium in Africa
Baruš V, Jarkovský J, Prokeš M (2007) Philometra ovata (Nematoda: Philometroidea): a potential sentinel species of heavy metal accumulation. Parasitol Res 100:929–933. doi:10.1007/s00436-006-0384-8
Bayoumy EM, Osman HAM, El-Bana LF, Hassanain MA (2008) Monogenean parasites as bioindicators for heavy metals status in some Egyptian Red Sea fishes. Glob Vet 2:117–122
Bergey L, Weis JS, Weis P (2002) Mercury uptake by the estuarine species Palaemonetes pugio and Fundulus heteroclitus compared with their parasites, Probopyrus pandalicola and Eustrongylides sp. Mar Pollut Bull 44:1046–1050. doi:10.1016/S0025-326X(02)00154-6
Billiard SM, Khan RA (2003) Chronic stress in cunner, Tautogolabrus adspersus, exposed to municipal and industrial effluents. Ecotoxicol Environ Saf 55:9–18. doi:10.1016/S0147-6513(02)00090-8
Biney C, Amuzu AT, Calamari D, Kaba N, Mbome IL, Naeve H, Ochumba PBO, Osibanjo O, Radegonde V, Saad MAH (1994) Review of heavy metals in the African aquatic environment. Ecotoxicol Environ Saf 28:134–159
Bird DJ, Duquesne S, Hoeksema SD, Langston WJ, Potter IC (2011) Complexity of spatial and temporal trends in metal concentrations in macroinvertebrate biomonitor species in the Severn Estuary and Bristol Channel. J Mar Biol Assoc U K 91:139–153. doi:10.1017/S0025315410001918
Blanar CA, Munkittrick KR, Houlahan J, MacLatchy DL, Marcogliese DJ (2009) Pollution and parasitism in aquatic animals: a meta-analysis of effect size. Aquat Toxicol 93:18–28. doi:10.1016/j.aquatox.2009.03.002
Blanar CA, Marcogliese DJ, Couillard C (2011) Natural and anthropogenic factors shape metazoan parasite community structure in mummichog (Fundulus heteroclitus) from two estuaries in New Brunswick, Canada. Folia Parasitol (Praha) 58:240–248. doi:10.14411/fp.2011.023
Blanco G, Frías O, Jiménez B, Gómez G (2003) Factors influencing variability and potential uptake routes of heavy metals in black kites exposed to emissions from a solid-waste incinerator. Environ Toxicol Chem 22:2711–2718. doi:10.1897/02-519
Blažek R, Jarkovský J, Koubková B, Gelnar M (2008) Seasonal variation in parasite occurrence and microhabitat distribution of monogenean parasites of gudgeon Gobio gobio (L.) Helminthologia 45:185–191. doi:10.2478/s11687-008-0037-9
Boggs JF, McMurry ST, Leslie DM, Engle DM, Lochmiller RL (1991) Influence of habitat modification on the community of gastrointestinal helminths of cotton rats. J Wildl Dis 27:584–593. doi:10.7589/0090-3558-27.4.584
Brabec J, Waeschenbach A, Scholz T, Littlewood DTJ, Kuchta R (2015) Molecular phylogeny of the Bothriocephalidea (Cestoda): molecular data challenge morphological classification. Int J Parasitol 45:761–771. doi:10.1016/j.ijpara.2015.05.006
Brasfield SM, Bradham K, Wells JB, Talent LG, Lanno RP, Janz DM (2004) Development of a terrestrial vertebrate model for assessing bioavailability of cadmium in the fence lizard (Sceloporus undulatus) and in ovo effects on hatchling size and thyroid function. Chemosphere 54:1643–1651. doi:10.1016/j.chemosphere.2003.09.030
Brázová T, Torres J, Eira C, Hanzelová V, Miklisová D, Šalamún P (2012) Perch and its parasites as heavy metal biomonitors in a freshwater environment: the case study of the Ružín Water Reservoir, Slovakia. Sensors 12:3068–3081. doi:10.3390/s120303068
Brázová T, Hanzelová V, Miklisová D, Šalamún P, Vidal-Martínez VM (2015) Host-parasite relationships as determinants of heavy metal concentrations in perch (Perca fluviatilis) and its intestinal parasite infection. Ecotoxicol Environ Saf 122:551–556. doi:10.1016/j.ecoenv.2015.09.032
Brito SV, Ferreira FS, Ribeiro SC, Anjos LA, Almeida WO, Mesquita DO, Vasconcellos A (2014) Spatial-temporal variation of parasites in Cnemidophorus ocellifer (Teiidae) and Tropidurus hispidus and Tropidurus semitaeniatus (Tropiduridae) from Caatinga areas in northeastern Brazil. Parasitol Res 113:1163–1169. doi:10.1007/s00436-014-3754-7
Broeg K, Zander S, Diamant A, Körting W, Krüner G, Paperna I, Westernhagen HV (1999) The use of fish metabolic, pathological and parasitological indices in pollution monitoring. I. North Sea. Helgol Mar Res 53:171–194. doi:10.1007/s101520050024
Brotheridge RM, Newton KE, Evans SW (1998) Presence of a parasitic nematode (Eustrongyloides sp.) in brown trout (Salmo trutta) from a heavy metal contaminated aquatic ecosystem. Chemosphere 37:2921–2934
Brown AF, Pascoe D (1989) Parasitism and host sensitivity to cadmium: an acanthocephalan infection of the freshwater amphipod Gammarus pulex. J Appl Ecol 26:473–487
Čadková Z, Miholová D, Száková J, Válek P, Jankovská I, Langrová I (2014) Is the tapeworm able to affect tissue Pb-concentrations in white rat? Parasitology 141:826–836. doi:10.1017/S0031182013002242
Cannicci S, Bartolini F, Dahdouh-Guebas F, Fratini S, Litulo C, Macia A, Mrabu EJ, Penha-Lopes G, Paula J (2009) Effects of urban wastewater on crab and mollusc assemblages in equatorial and subtropical mangroves of East Africa. Estuar Coast Shelf Sci 84:305–317. doi:10.1016/j.ecss.2009.04.021
Chang ACG, Flores MJC (2015) Morphology and viability of adult Fasciola gigantica (giant liver flukes) from Philippine carabaos (Bubalus bubalis) upon in vitro exposure to lead. Asian Pac J Trop Biomed 5:493–496. doi:10.1016/j.apjtb.2015.03.008
Chapman LJ, Lanciani CA, Chapman CA (2000) Ecology of a diplozoon parasite on the gills of the African cyprinid Barbus neumayeri. Afr J Ecol 38:312–320. doi:10.1046/j.1365-2028.2000.00252.x
Chen HY, Cheng YS, Grabner DS, Chang SH, Shih HH (2014) Effect of different temperatures on the expression of the newly characterized heat shock protein 90 (Hsp90) in L3 of Anisakis spp. isolated from Scomber australasicus. Vet Parasitol 205:540–550. doi:10.1016/j.vetpar.2014.09.013
Chibani M, Ziólkowska M, Kijewska A, Rokicki J (2001) Pomphorhynchus laevis parasite of flounder Platichthys flesus as a biological indicator of pollution in the Baltic Sea. J Mar Biol Assoc U K 81:165–166. doi:10.1017/S0025315401003514
Claassens L, Dahms S, van Vuren JHJ, Greenfield R (2016) Artificial mussels as indicators of metal pollution in freshwater systems: a field evaluation in the Koekemoer Spruit, South Africa. Ecol Indic 60:940–946. doi:10.1016/j.ecolind.2015.08.047
Crafford D, Avenant-Oldewage A (2009) Application of a fish health assessment index and associated parasite index to Clarias gariepinus (Teleostei: Clariidae) in the Vaal River system, South Africa. Afr J Aquat Sci 34:261–272. doi:10.2989/AJAS.2009.34.3.8.984
Crafford D, Avenant-Oldewage A (2010) Bioaccumulation of non-essential trace metals in tissues and organs of Clarias gariepinus (sharptooth catfish) from the Vaal River system—strontium, aluminium, lead and nickel. Water SA 36:621–640. doi:10.4314/wsa.v36i5.61996
Crafford D, Avenant-Oldewage A (2011) Uptake of selected metals in tissues and organs of Clarias gariepinus (sharptooth catfish) from the Vaal River system—chromium, copper, iron, manganese and zinc. Water SA 37:181–200. doi:10.4314/wsa.v37i2.65864
Crafford D, Luus-Powell W, Avenant-Oldewage A (2014) Monogenean parasites from fishes of the Vaal Dam, Gauteng Province, South Africa I. Winter survey versus summer survey comparison from Labeo capensis (Smith 1841) and Labeo umbratus (Smith, 1841) hosts. Acta Parasitol 59:17–24. doi:10.2478/s11686-014-0205-7-x
Craig S, Overnell J (2003) Metals in squid, Loligo forbesi, adults, eggs and hatchlings. No evidence for a role for Cu- or Zn-metallothionein. Comp Biochem Physiol - C Toxicol Pharmacol 134:311–317. doi:10.1016/S1532-0456(02)00274-0
Cross MA, Irwin SW, Fitzpatrick SM (2001) Effects of heavy metal pollution on swimming and longevity in cercariae of Cryptocotyle lingua (Digenea: Heterophyidae). Parasitology 123:499–507. doi:10.1017/S0031182001008708
Dahms S, Baker NJ, Greenfield R (2017) Ecological risk assessment of trace elements in sediment: a case study from Limpopo, South Africa. Ecotoxicol Environ Saf 135:106–114. doi:10.1016/j.ecoenv.2016.09.036
de Buron I, James E, Riggs-Gelasco P, Ringwood AH, Rolando E, Richardson D (2009) Overview of the status of heavy metal accumulation by helminths with a note on the use of in vitro culture of adult acanthocephalans to study the mechanisms of bioaccumulation. Neotrop Helminthol 3:101–110
Degger N, Avenant-Oldewage A, Greenfield R (2009) Innovative fluorescence detection technique for metals in cestode egg-shells. Afr Zool 44:204–207. doi:10.3377/004.044.0208
Degger N, Wepener V, Richardson BJ, Wu RSS (2011a) Brown mussels (Perna perna) and semi-permeable membrane devices (SPMDs) as indicators of organic pollutants in the South African marine environment. Mar Pollut Bull 63:91–97. doi:10.1016/j.marpolbul.2011.04.024
Degger N, Wepener V, Richardson BJ, Wu RSS (2011b) Application of artificial mussels (AMs) under South African marine conditions: a validation study. Mar Pollut Bull 63:108–118. doi:10.1016/j.marpolbul.2011.04.040
Department of Water Affairs and Forestry (1996) South African Water Quality Guidelines. Volume 7: aquatic ecosystems
Dickens CWS, Graham PM (2002) The South African Scoring System (SASS) version 5 rapid bioassessment method for rivers. Afr J Aquat Sci 27:1–10. doi:10.2989/16085914.2002.9626569
Dos Santos QM, Avenant-Oldewage A (2016) The description of a new diplozoid species, Paradiplozoon krugerense n. sp., from Labeo rosae Steindachner, 1894 and Labeo congoro Peters, 1852 in the Kruger National Park, South Africa with notes on the effect of water quality on its infection variables. Hydrobiologia 777:225–241. doi:10.1007/s10750-016-2776-9
Dušek L, Gelnar M, Šebelová Š (1998) Biodiversity of parasites in a freshwater environment with respect to pollution: metazoan parasites of chub (Leuciscus cephalus L.) as a model for statistical evaluation. Int J Parasitol 28:1555–1571. doi:10.1016/S0020-7519(98)00130-1
Dzika E, Kuształa A, Kuształa M (2007) Parasites of carp bream, Abramis brama, from Lake Jamno, Poland. Helminthologia 44:222–225
Dzikowski R, Paperna I, Diamant A (2003) Use of fish parasite species richness indices in analyzing anthropogenically impacted coastal marine ecosystems. Helgol Mar Res 57:220–227. doi:10.1007/s10152-003-0138-2
Eira C, Torres J, Vingada J, Miquel J (2005) Concentration of some toxic elements in Oryctolagus cuniculus and in its intestinal cestode Mosgovoyia ctenoides, in Dunas de Mira (Portugal). Sci Total Environ 346:81–86. doi:10.1016/j.scitotenv.2004.11.014
Eira C, Torres J, Miquel J, Vaqueiro J, Soares AMVM, Vingada J (2009) Trace element concentrations in Proteocephalus macrocephalus (Cestoda) and Anguillicola crassus (Nematoda) in comparison to their fish host, Anguilla anguilla in Ria de Aveiro, Portugal. Sci Total Environ 407:991–998. doi:10.1016/j.scitotenv.2008.10.040
Faulkner BC, Lochmiller RL (2000) Ecotoxicity revealed in parasite communities of Sigmodon hispidus in terrestrial environments contaminated with petrochemicals. Environ Pollut 110:135–145. doi:10.1016/S0269-7491(99)00276-6
Förstner U, Prosi F (1979) Heavy metal pollution in freshwater ecosystems. In: Ravera O (ed) Biological aspects of freshwater pollution. Pergamon, Oxford, pp 129–214
Frank SN, Godehardt S, Nachev M, Trubiroha A, Kloas W, Sures B (2013) Influence of the cestode Ligula intestinalis and the acanthocephalan Polymorphus minutus on levels of heat shock proteins (HSP70) and metallothioneins in their fish and crustacean intermediate hosts. Environ Pollut 180:173–179. doi:10.1016/j.envpol.2013.05.014
Galli P, Crosa G, Mariniello L, Ortis M, D’Amelio S (2001) Water quality as a determinant of fish parasite communities. Hydrobiologia 452:173–179
Gao Q, Nie P (2000) Lead content in the monogenean Ancryocephalus mogurndae and in different organs of its host, the mandarin fish, Siniperca chuatsi. China Environ Sci 20:233–236
Gelnar M (1991) Experimental verification of the effect of constant and changing water temperature on the micropopulation growth in Gyrodactylus gobiensis Gläser, 1974 (Monogenea) parasitizing Gudgeon (Gobio gobio L.) Folia Parasitol (Praha) 38:123–131
Gheorghiu C, Cable J, Marcogliese DJ, Scott ME (2007) Effects of waterborne zinc on reproduction, survival and morphometrics of Gyrodactylus turnbulli (Monogenea) on guppies (Poecilia reticulata). Int J Parasitol 37:375–381. doi:10.1016/j.ijpara.2006.09.004
Gheorgiu C, Marcogliese DJ, Scott M (2006) Concentration-dependent effects of waterborne zinc on population dynamics of Gyrodactylus turnbulli (Monogenea) on isolated guppies (Poecilia reticulata). Parasitology 132:225–232. doi:10.1017/S003118200500898X
Gilbert BM, Avenant-Oldewage A (2016a) Seasonal occurrence and microhabitat specificity of Paradiplozoon ichthyoxanthon Avenant-Oldewage in Avenant-Oldewage et al., 2014 (Monogenea: Diplozoidae) infecting Labeobarbus aeneus (Burchell) (Teleostei: Cyprinidae) from the Vaal Dam, South Africa: water quality and host size as determining factors? Folia Parasitol (Praha) 63:4. doi:10.14411/fp.2016.004
Gilbert BM, Avenant-Oldewage A (2016b) Effects of altered water quality and trace elements on the infection variables of Paradiplozoon ichthyoxanthon (Monogenea: Diplozoidae) from two sites in the Vaal River system, South Africa. Acta Parasitol 61:52–62. doi:10.1515/ap-2016-0005
Gilbert BM, Avenant-Oldewage A (2016c) Hatchability and survival of oncomiracidia of Paradiplozoon ichthyoxanthon (Monogenea: Diplozoidae) exposed to aqueous aluminium. Parasit Vectors 9:420
Gilbert BM, Avenant-Oldewage A (2017) Metal sequestration in vitellaria and sclerites, and reactive oxygen intermediates in a freshwater monogenean, Paradiplozoon ichthyoxanthon. PLoS One 12:e0177558. doi:10.1371/journal.pone.0177558
Goodyear KL, McNeill S (1999) Bioaccumulation of heavy metals by aquatic macro-invertebrates of different feeding guilds: a review. Sci Total Environ 229:1–19. doi:10.1016/S0048-9697(99)00051-0
Granath W, Esch GW (1983) Temperature and other factors that regulate the composition and infrapopulation densities of Bothriocephalus acheilognathi (Cestoda) in Gambusia affinis (Pisces). J Parasitol 69:1116–1124
Greenfield R, Wepener V, Degger N, Brink K (2011) Richards Bay Harbour: metal exposure monitoring over the last 34 years. Mar Pollut Bull 62:1926–1931. doi:10.1016/j.marpolbul.2011.04.026
Greenfield R, Brink K, Degger N, Wepener V (2014) The usefulness of transplantation studies in monitoring of metals in the marine environment: South African experience. Mar Pollut Bull 85:566–573. doi:10.1016/j.marpolbul.2014.03.032
Hagen AG, Hytterød S, Olstad K (2014) Low concentrations of sodium hypochlorite affect population dynamics in Gyrodactylus salaris (Malmberg, 1957): practical guidelines for the treatment of the Atlantic salmon, Salmo salar L. parasite. J Fish Dis 37:1003–1011. doi:10.1111/jfd.12218
Hakalahti T, Valtonen ET (2003) Population structure and recruitment of the ectoparasite Argulus coregoni Thorell (Crustacea: Branchiura) on a fish farm. Parasitology 127:79–85. doi:10.1017/S0031182003003196
Halmetoja A, Valtonen ET, Koskenniemi E (2000) Perch (Perca fluviatilis L.) parasites reflect ecosystem conditions: a comparison of a natural lake and two acidic reservoirs in Finland. Int J Parasitol 30:1437–1444. doi:10.1016/S0020-7519(00)00115-6
Hanzelova V (1992) Proteocephalus neglectus as a possible indicator of changes in the ecological balance of aquatic environments. J Helminthol 66:17–24. doi:10.1017/S0022149X00012517
Harding WR, Taylor JC (2014) Diatoms as indicators of historical water quality: a comparison of samples taken in the Wemmershoek catchment (Western Province, South Africa) in 1960 and 2008. Water SA 40:601–606. doi:10.4314/wsa.v40i4.4
Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, Hofmann EE, Lipp EK, Osterhaus ADME, Overstreet RM, Porter JW, Smith GW, Vasta GR (1999) Emerging marine diseases: climate links and anthropogenic factors. Science 285:1505–1510. doi:10.1126/science.285.5433.1505
Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD (2002) Climate warming and disease risks for terrestrial and marine biota. Science 296:2158–2162. doi:10.1126/science.1063699
Hayes ML, Bonaventura J, Mitchell TP, Prospero JM, Shinn EA, Van Dolah F, Barber RT (2001) How are climate and marine biological outbreaks functionally linked? Hydrobiologia 460:213–220
Heinonen J, Kukkonen JVK, Holopainen IJ (2000) Toxicokinetics of 2,4,5-trichlorophenol and benzo(a)pyrene in the clam Pisidium amnicum: effects of seasonal temperatures and trematode parasites. Arch Environ Contam Toxicol 39:352–359. doi:10.1007/s002440010115
Hodson PV (1988) The effect of metal metabolism on uptake, disposition and toxicity in fish. Aquat Toxicol 11:3–18
Hofmann H, Blasco-Costa I, Knudsen R, Matthaei CD, Valois A, Lange K (2016) Parasite prevalence in an intermediate snail host is subject to multiple anthropogenic stressors in a New Zealand river system. Ecol Indic 60:84–852. doi:10.1016/j.ecolind.2015.08.022
Höglund J, Thulin J (1989) Thermal effects on the seasonal dynamics of Paradiplozoon homoion (Bychowsky & Nagibina, 1959) parasitizing roach, Rutilus rutilus (L.) J Helminthol 63:93. doi:10.1017/S0022149X0000883X
Holt EA, Miller SW (2010) Bioindicators: using organisms to measure environmental impacts. Nat Educ Knowl 3:8. doi:10.5962/bhl.title.62081
Hook SE, Fisher NS (2001) Reproductive toxicity of metals in calanoid copepods. Mar Biol 138:1131–1140. doi:10.1007/s002270000533
Hu H (2000) Exposure to metals. Prim Care - Clin Off Pract 27:983–996. doi:10.1016/S0095-4543(05)70185-8
Jakob E, Hanel R, Klimpel S, Zumholz K (2008) Salinity dependence of parasite infestation in the European eel Anguilla anguilla in northern Germany. ICES J Mar Sci 66:358–366. doi:10.1093/icesjms/fsn160
Jankovská I, Vadlejch J, Száková J, Miholová D, Kunc P, Knížková I, Čadková Z, Langrová I (2010) Experimental studies on the cadmium accumulation in the cestode Moniezia expansa (Cestoda: Anoplocephalidae) and its final host (Ovis aries). Exp Parasitol 126:130–134. doi:10.1016/j.exppara.2010.04.010
Jeffree RA, Oberhansli F, Teyssie J-L (2008) The accumulation of lead and mercury from seawater and their depuration by eggs of the spotted dogfish Scyliorhinus canicula (Chondrichthys). Arch Environ Contam Toxicol 55:451–461
Jeney Z, Valtonen ET, Jeney G, Jokinen EI (2002) Effect of pulp and paper mill effluent (BKME) on physiological parameters of roach (Rutilus rutilus) infected by the digenean Rhipidocotyle fennica. Folia Parasitol (Praha) 49:103–108
Jirsa F, Leodolter-Dvorak M, Krachler R, Frank C (2008) Heavy metals in the nase, Chondrostoma nasus (L. 1758), and its intestinal parasite Caryophyllaeus laticeps (Pallas 1781) from Austrian rivers: bioindicative aspects. Arch Environ Contam Toxicol 55:619–626. doi:10.1007/s00244-008-9154-1
Jirsa F, Konecny R, Frank C, Sures B (2011) The parasite community of the nase Chondrostoma nasus (L. 1758) from Austrian rivers. J Helminthol 85:255–262. doi:10.1017/S0022149X10000490
Kádár E, Costa V, Santos RS, Powell JJ (2006) Tissue partitioning of micro-essential metals in the vent bivalve Bathymodiolus azoricus and associated organisms (endosymbiont bacteria and a parasite polychaete) from geochemically distinct vents of the Mid-Atlantic Ridge. J Sea Res 56:45–52. doi:10.1016/j.seares.2006.01.002
Karvonen A, Bagge AM, Valtonen ET (2005) Parasite assemblages of crucian carp (Carassius carassius)—is depauperate composition explained by lack of parasite exchange, extreme environmental conditions or host unsuitability? Parasitology 131:273–278. doi:10.1017/S0031182005007572
Keppel M, Dangel KC, Sures B (2014) Comparison of infection success, development and swim bladder pathogenicity of two congeneric Anguillicola species in experimentally infected Anguilla anguilla and A. japonica. Parasitol Res 113:3727–3735. doi:10.1007/s00436-014-4038-y
Keppel M, Dangel KC, Sures B (2016) The Hsp70 response of Anguillicola species to host-specific stressors. Parasitol Res 115:2149–2154. doi:10.1007/s00436-016-4956-y
Khalil M, Furness D, Polwart A, Hoole D (2009) X-ray microanalysis (EDXMA) of cadmium-exposed eggs of Bothriocephalus acheilognathi (Cestoda: Bothriocephalidea) and the influence of this heavy metal on coracidial hatching and activity. Int J Parasitol 39:1093–1098. doi:10.1016/j.ijpara.2009.02.023
Khan RA, Kiceniuk J (1983) Effects of crude oils on the gastrointestinal parasites of two species of marine fish. J Wildl Dis 19:253–258
Khan RA, Kiceniuk JW (1988) Effect of petroleum aromatic hydrocarbons on monogeneids parasitizing Atlantic cod, Gadus morhua L. Bull Environ Contam Toxicol 41:94–100
Khan AR, Rasheed M (1993) Influence of season on the intensity of helminth infection in Schizothorax esocinus heckel from the Wular Lake. Indian J Helminthol 45:64–73
Khan RA, Thulin J (1991) Influence of pollution on parasites of aquatic animals. Adv Parasitol 30:201–239
Khan RA, Barker DE, Williams-Ryan K, Hooper RG (1994) Influence of crude oil and pulp and paper mill effluent on mixed infections of Trichodina cottidarium and T. saintjohnsi (Ciliophora) parasitizing Myoxocephalus octodecemspinosus and M. scorpius. Can J Zool 72:247–251
Knight DP, Feng D, Stewart M (1996) Structure and function of the selachian egg case. Biol Rev 71:81–91
Koob TJ (1991) Deposition and binding of calcium and magnesium in egg capsules of Raja erinacea Mitchill during formation and tanning in utero. Copeia 1991:339–347
Koskivaara M, Valtonen ET (1991) Paradiplozoon homoion (Monogenea) and some other gill parasites on roach Rutilus rutilus in Finland. Aqua Fenn 21:137–143
Koskivaara M, Valtonen ET, Prost M (1991) Seasonal occurrence of gyrodactylid monogeneans on the roach (Rutilus rutilus) and variations between four lakes of differing water quality in Finland. Aqua Fenn 21:47–55. doi:10.1016/0020-7519(91)90061-B
Koskivaara M, Valtonen ET, Vuori KM (1992) Microhabitat distribution and coexistence of Dactylogyrus species (Monogenea) on the gills of roach. Parasitology 104:273–281. doi:10.1017/S0031182000061710
Kua BC, Choong FC, Leaw YY (2013) Effect of salinity and temperature on marine leech, Zeylanicobdella arugamensis (De Silva) under laboratory conditions. J Fish Dis 37:201–207. doi:10.1111/jfd.12087
Kuperman BI (1992) Fish parasites as biological indicators of pollution of water bodies. Parazitologiya 26:479–482
Lafferty KD (1997) Reviews environmental parasitology: what can parasites tell us about human impacts on the environment? Parasitol Today 13:251–255
Lamková K, Šimková A, Palíková M, Jurajda P, Lojek A (2007) Seasonal changes of immunocompetence and parasitism in chub (Leuciscus cephalus), a freshwater cyprinid fish. Parasitol Res 101:775–789. doi:10.1007/s00436-007-0546-3
le Roux LE, Avenant-Oldewage A, van der Walt FC (2011) Aspects of the ecology of Cichlidogyrus philander collected from Pseudocrenilabrus philander philander from the Padda Dam, Gauteng, South Africa. Afr Zool 46:103–116. doi:10.1080/15627020.2011.11407484
Le TTY, Rijsdijk L, Sures B, Jan Hendriks A (2014) Accumulation of persistent organic pollutants in parasites. Chemosphere 108:145–151. doi:10.1016/j.chemosphere.2014.01.036
Le TTY, Nachev M, Grabner D, Hendriks AJ, Sures B (2016) Development and validation of a biodynamic model for mechanistically predicting metal accumulation in fish-parasite systems. PLoS ONE 11(8):e01610901. doi:10.1371/journal.pone.0161091
Lefcort H, Aguon MQ, Bond KA, Chapman KR, Chaquette R, Clark J, Kornachuk P, Lang BZ, Martin JC (2002) Indirect effects of heavy metals on parasites may cause shifts in snail species compositions. Arch Environ Contam Toxicol 43:34–41. doi:10.1007/s00244-002-1173-8
Leonard EM, Wood CM (2013) Acute toxicity, critical body residues, Michaelis-Menten analysis of bioaccumulation, and ionoregulatory disturbance in response to waterborne nickel in four invertebrates: Chironomus riparius, Lymnaea stagnalis, Lumbriculus variegatus and Daphnia pulex. Comp Biochem Physiol - C Toxicol Pharmacol 158:10–21. doi:10.1016/j.cbpc.2013.03.008
Lins DC, Meirelles ME, Malm O, Lima NRW (2008) Mercury concentration in the freshwater bonefish Cyphocharax gilbert (Curimatidae) and its parasite the crustacean Riggia paranensis (Cymothoidae). Neotrop Ichthyol 6:283–288. doi:10.1590/S1679-62252008000200017
Lõhmus M, Björklund M (2015) Climate change: what will it do to fish–parasite interactions? Biol J Linn Soc 116:397–411. doi:10.1111/bij.1284
MacDonald S, Jones A (1978) Egg-laying and hatching rhythms in the monogenean Diplozoon homoion gracile from the southern barbel (Barbus meridionalis). J Helminthol 52:23–28
MacKenzie K (1999) Parasites as pollution indicators in marine ecosystems: a proposed early warning system. Mar Pollut Bull 38:955–959. doi:10.1016/S0025-326X(99)00100-9
MacLeod CD, Poulin R (2012) Host-parasite interactions: a litmus test for ocean acidification? Trends Parasitol 28:365–369. doi:10.1016/j.pt.2012.06.007
Madanire-Moyo GN, Barson M (2010) Diversity of metazoan parasites of the African catfish Clarias gariepinus (Burchell, 1822) as indicators of pollution in a subtropical African river system. J Helminthol 84:216–227. doi:10.1017/S0022149X09990563
Madanire-Moyo GN, Luus-Powell WJ, Olivier PAS (2012) Diversity of metazoan parasites of the Mozambique tilapia, Oreochromis mossambicus (Peters, 1852), as indicators of pollution in the Limpopo and Olifants River systems. Onderstepoort J Vet Res 79:1–9. doi:10.4102/ojvr.v79i1.362
Madoni P, Romeo MG (2006) Acute toxicity of heavy metals towards freshwater ciliated protists. Environ Pollut 141:1–7. doi:10.1016/j.envpol.2005.08.025
Mahmoud KMA, Abu Taleb HMA (2013) Fresh water snails as bioindicator for some heavy metals in the aquatic environment. Afr J Ecol 51:193–198. doi:10.1111/aje.12019
Malek M, Haseli M, Mobedi I, Ganjali MR, MacKenzie K (2007) Parasites as heavy metal bioindicators in the shark Carcharhinus dussumieri from the Persian Gulf. Parasitology 134:1053–1056. doi:10.1017/S0031182007002508
Marcogliese DJ (2001) Implications of climate change for parasitism of animals in the aquatic environment. Can J Zool 79:1331–1352. doi:10.1139/z01-067
Marcogliese DJ (2004) Parasites: small players with crucial roles in the ecological theater. EcoHealth 1:151–164. doi:10.1007/s10393-004-0028-3
Marcogliese DJ (2005) Parasites of the superorganism: are they indicators of ecosystem health? Int J Parasitol 35:705–716. doi:10.1016/j.ijpara.2005.01.015
Marcogliese DJ (2008) The impact of climate change on the parasites and infectious diseases of aquatic animals. Rev Sci Tech Int Off Epizoot 27:467–484
Marcogliese DJ (2016) The distribution and abundance of parasites in aquatic ecosystems in a changing climate: more than just temperature. Integr Comp Biol 56:611–619. doi:10.1093/icb/icw036
Marcogliese DJ, Pietrock M (2011) Combined effects of parasites and contaminants on animal health: parasites do matter. Trends Parasitol 27:123–130. doi:10.1016/j.pt.2010.11.002
Marcogliese DJ, Nagler JJ, Cyr DG (1998) Effects of exposure to contaminated sediments on the parasite fauna of American plaice (Hippoglossoides platessoides). Bull Environ Contam Toxicol 61:88–95. doi:10.1007/s001289900733
Marcos-López M, Gale P, Oidtmann BC, Peeler EJ (2010) Assessing the impact of climate change on disease emergence in freshwater fish in the United Kingdom. Transbound Emerg Dis 57:293–304. doi:10.1111/j.1865-1682.2010.01150.x
Martins CDMG, Barcarolli IF, de Menezes EJ, Giacomin MM, Wood CM, Bianchini A (2011) Acute toxicity, accumulation and tissue distribution of copper in the blue crab Callinectes sapidus acclimated to different salinities: in vivo and in vitro studies. Aquat Toxicol 101:88–99. doi:10.1016/j.aquatox.2010.09.005
Marx HM (1996) Evaluation of a health assessment index with reference to metal bioaccumulation in Clarias gariepinus and aspects of the biology of the parasite Lamproglena clariae. University of Johannesburg, Johannesburg
Mazhar R, Shazili NA, Harrison FS (2014) Comparative study of the metal accumulation in Hysterothalycium reliquens (nematode) and Paraphilometroides nemipteri (nematode) as compared with their doubly infected host, Nemipterus peronii (notched threadfin bream). Parasitol Res 113:3737–3743. doi:10.1007/s00436-014-4039-x
Mbokane EM, Matla MM, Theron J, Luus-Powell WJ (2015) Seasonal dynamics and occurrences of three Dactylogyrus species on the gills of three cyprinids at Nwanedi – Luphephe dams in Limpopo province, South Africa. Afr Zool 50:119–125. doi:10.1080/15627020.2015.1021175
Mehta A, Shaha C (2006) Mechanism of metalloid-induced death in Leishmania spp.: role of iron, reactive oxygen species, Ca2+, and glutathione. Free Radic Biol Med 40:1857–1868. doi:10.1016/j.freeradbiomed.2006.01.024
Mhlanga W (2000) Mercury in tigerfsh (Hydrocynus vittatus, Castelnau), green happy (Sargochromis codringtonii, Boulenger) and kapenta (Limnothrissa miodon, Boulenger) from Lake Kariba, Zimbabwe. Afr J Ecol 38:224–229
Minguez L, Molloy DP, Guérold F, Giambérini L (2011) Zebra mussel (Dreissena polymorpha) parasites: potentially useful bioindicators of freshwater quality? Water Res 45:665–673. doi:10.1016/j.watres.2010.08.028
Morley NJ, Crane M, Lewis JW (2001) Toxicity of cadmium and zinc to miracidia of Schistosoma mansoni. Parasitology 122(Pt 1):81–85. doi:10.1017/S0031182000007083
Morley NJ, Crane M, Lewis JW (2003) Cadmium toxicity and snail-digenean interactions in a population of Lymnaea spp. J Helminthol 77:49–55. doi:10.1079/JOH2002148
Morley NJ, Crane M, Lewis JW (2004) Influence of cadmium exposure on the incidence of first intermediate host encystment by Echinoparyphium recurvatum cercariae in Lymnaea peregra. J Helminthol 78:329–332. doi:10.1079/JOH2004267
Morley NJ, Crane M, Lewis JW (2005a) Toxicity of cadmium and zinc mixtures to cercarial tail loss in Diplostomum spathaceum (Trematoda:Diplostomidae). Ecotoxicol Environ Saf 60:53–60. doi:10.1016/j.ecoenv.2003.12.018
Morley NJ, Crane M, Lewis JW (2005b) Changes in survival characteristics of Diplostomum spathaceum cercariae emerged from cadmium-exposed Lymnaea stagnalis. J Helminthol 79:55–59. doi:10.1079/JOH2004271
Morley NJ, Costa HH, Lewis JW (2010) Effects of a chemically polluted discharge on the relationship between fecundity and parasitic infections in the chub (Leuciscus cephalus) from a river in southern England. Arch Environ Contam Toxicol 58:783–792. doi:10.1007/s00244-009-9386-8
Morris T, Avenant-Oldewage A, Lamberth S, Reed C (2016) Shark parasites as bio-indicators of metals in two South African embayments. Mar Pollut Bull. doi:10.1016/j.marpolbul.2016.01.027
Nachev M, Sures B (2009) The endohelminth fauna of barbel (Barbus barbus) correlates with water quality of the Danube River in Bulgaria. Parasitology 136:545–552. doi:10.1017/S003118200900571X
Nachev M, Sures B (2016) Seasonal profile of metal accumulation in the acanthocephalan Pomphorhynchus laevis: a valuable tool to study infection dynamics and implications for metal monitoring. Parasit Vectors 9:300. doi:10.1186/s13071-016-1576-4
Nachev M, Zimmermann S, Rigaud T, Sures B (2010) Is metal accumulation in Pomphorhynchus laevis dependent on parasite sex or infrapopulation size? Parasitology 137:1239–1248. doi:10.1017/S0031182010000065
Nomquphu W, Braune E, Mitchell S (2007) The changing water resources monitoring environment in South Africa. S Afr J Sci 103:306–310
Ogut H, Palm HW (2005) Seasonal dynamics of Trichodina spp. on whiting (Merlangius merlangus) in relation to organic pollution on the eastern Black Sea coast of Turkey. Parasitol Res 96:149–153. doi:10.1007/s00436-005-1346-2
Olivier PAS, Luus-Powell WJ, Saayman JE (2009) Report on some monogenean and clinostomid infestations of freshwater fish and waterbird hosts in Middle Letaba Dam, Limpopo Province, South Africa. Onderstepoort J Vet Res 76:187–199
Otachi EO, Körner W, Avenant-Oldewage A, Fellner-Frank C, Jirsa F (2014) Trace elements in sediments, blue spotted tilapia Oreochromis leucostictus (Trewavas, 1933) and its parasite Contracaecum multipapillatum from Lake Naivasha, Kenya, including a comprehensive health risk analysis. Environ Sci Pollut Res 21:7339–7349. doi:10.1007/s11356-014-2602-8
Otachi EO, Szostakowska B, Jirsa F, Fellner-Frank C (2015) Parasite communities of the elongate tigerfish Hydrocynus forskahlii (Cuvier 1819) and redbelly tilapia Tilapia zillii (Gervais 1848) from Lake Turkana, Kenya: influence of host sex and size. Acta Parasitol 60:9–20. doi:10.1515/ap-2015-0002
Oyoo-Okoth E, Admiraal W, Osano O, Hoitinga L, Kraak MHS (2010) Metal specific partitioning in a parasite-host assemblage of the cestode Ligula intestinalis and the cyprinid fish Rastrineobola argentea. Sci Total Environ 408:1557–1562. doi:10.1016/j.scitotenv.2009.11.054
Palm HW, Dobberstein RC (1999) Occurrence of trichodinid ciliates (Peritricha: Urceolariidae) in the Kiel Fjord, Baltic Sea, and its possible use as a biological indicator. Parasitol Res 85:726–732. doi:10.1007/s004360050622
Paperna I (1996) Parasites, infections and diseases of fishes in Africa—an update. CIF Technical Paper. No. 31. Rome
Paul-Pont I, De Montaudouin X, Gonzalez P, Jude F, Raymond N, Paillard C, Baudrimont M (2010a) Interactive effects of metal contamination and pathogenic organisms on the introduced marine bivalve Ruditapes philippinarum in European populations. Environ Pollut 158:3401–3410. doi:10.1016/j.envpol.2010.07.028
Paul-Pont I, Gonzalez P, Baudrimont M, Jude F, Raymond N, Bourrasseau L, Le Goïc N, Haynes F, Legeay A, Paillard C, de Montaudouin X (2010b) Interactive effects of metal contamination and pathogenic organisms on the marine bivalve Cerastoderma edule. Mar Pollut Bull 60:515–525. doi:10.1016/j.marpolbul.2009.11.013
Pech D, Vidal-Martínez VM, Aguirre-Macedo ML, Gold-Bouchot G, Herrera-Silveira J, Zapata-Pérez O, Marcogliese DJ (2009) The checkered puffer (Spheroides testudineus) and its helminths as bioindicators of chemical pollution in Yucatan coastal lagoons. Sci Total Environ 407:2315–2324. doi:10.1016/j.scitotenv.2008.11.054
Pečínková M, Matějusová I, Koubková B, Gelnar M (2005) Classification and occurrence of abnormally developed Paradiplozoon homoion (Monogenea, Diplozoinae) parasitising gudgeon Gobio gobio. Dis Aquat Org 64:63–68. doi:10.3354/dao064063
Pettersen RA, Vøllestad LA, Flodmark LEW, Poléo ABS (2006) Effects of aqueous aluminium on four fish ectoparasites. Sci Total Environ 369:129–138. doi:10.1016/j.scitotenv.2006.05.024
Pietrock M, Meinelt T, Marcogliese DJ (2008) Effects of cadmium exposure on embryogenesis of Stagnicola elodes (Mollusca, Gastropoda): potential consequences for parasite transmission. Arch Environ Contam Toxicol 55:43–48. doi:10.1007/s00244-007-9083-4
Pilecka-Rapacz M, Piasecki W, Czerniawski R, Sługocki Ł, Krepski T, Domagała J (2015) The effect of warm discharge waters of a power plant on the occurrence of parasitic Metazoa in freshwater bream, Abramis brama (L.) Bull Eur Assoc Fish Pathol 35:94–103
Poléo ABS, Schjolden J, Hansen H, Bakke TA, Mo TA, Rosseland BO, Lydersen E (2004) The effect of various metals on Gyrodactylus salaris (Platyhelminthes, Monogenea) infections in Atlantic salmon (Salmo salar). Parasitology 128:169–177. doi:10.1017/S0031182003004396
Poulin R (1995) Phylogeny, ecology, and the richness of parasite communities in vertebrates. Ecol Monogr 65:283–302
Poulin R, Blanar CA, Thieltges DW, Marcogliese DJ (2011) The biogeography of parasitism in sticklebacks: distance, habitat differences and the similarity in parasite occurrence and abundance. Ecography (Cop) 34:540–551. doi:10.1111/j.1600-0587.2010.06826.x
Raymond KMN, Chapman L, Lanciani CA (2006) Host, macrohabitat, and microhabitat specificity in the gill parasite Afrodiplozoon polycotyleus (Monogenea). J Parasitol 92:1211–1217. doi:10.1645/GE-621R.1
Reed C, MacKenzie K, Van der Lingen CD (2012) Parasites of South African sardines, Sardinops sagax, and an assessment of their potential as biological tags. Bull Eur Assoc Fish Pathol 32:41–48
Retief NR, Avenant-Oldewage A, Du Preez H (2006) The use of cestode parasites from the largemouth yellowfish, Labeobarbus kimberleyensis (Gilchrist and Thompson, 1913) in the Vaal Dam, South Africa as indicators of heavy metal bioaccumulation. Phys Chem Earth 31:840–847. doi:10.1016/j.pce.2006.08.004
Retief NR, Avenant-Oldewage A, Du Preez HH (2007) Ecological aspects of the occurrence of Asian tapeworm, Bothriocephalus acheilognathi Yamaguti, 1934 infection in the largemouth yellowfish, Labeobarbus kimberleyensis (Gilchrist and Thompson, 1913) in the Vaal Dam, South Africa. Phys Chem Earth 32:1384–1390. doi:10.1016/j.pce.2007.07.044
Retief NR, Avenant-Oldewage A, Du Preez HH (2009) Seasonal study on Bothriocephalus as indicator of metal pollution in yellowfish, South Africa. Water SA 35:315–322
Richards MP (1997) Trace mineral metabolism in the avian embryo. Poult Sci 76:152–164. doi:10.1093/ps/76.1.152
Riggio M, Trinchella F, Filosa S, Parisi E, Scudiero R (2003) Accumulation of zinc, copper, and metallothionein mRNA in lizard ovary proceeds without a concomitant increase in metallothionein content. Mol Reprod Dev 66:374–382. doi:10.1002/mrd.10365
Riggs MR, Lemly AD, Esch GW (1987) The growth, biomass, and fecundity of Bothriocephalus acheilognathi in a North Carolina cooling reservoir. J Parasitol 73:893–900
Robinson SA, Forbes MR, Hebert CE (2010) Mercury in parasitic nematodes and trematodes and their double-crested cormorant hosts: bioaccumulation in the face of sequestration by nematodes. Sci Total Environ 408:5439–5444. doi:10.1016/j.scitotenv.2010.07.071
Rohde K (1984) Ecology of marine parasites. Helgoländer Meeresun 37:5–33
Rosa IC, Raimundo J, Lopes VM, Brandão C, Couto A, Santos C, Cabecinhas AS, Cereja R, Calado R, Caetano M, Rosa R (2015) Cuttlefish capsule: an effective shield against contaminants in the wild. Chemosphere 135:7–13. doi:10.1016/j.chemosphere.2015.03.050
Roux DJ, van Vliet HR, van Veelen M (1993) Towards integrated water quality monitoring: assessment of ecosystem health. Water SA 19:275–280
Sabo R, Sabová L, Legáth J (2009) The use of parasites as bioindicators of pesticide exposure. Interdiscip Toxicol 2:187–189. doi:10.2478/v10102-009-0015-1
Saliu JK, Akinsanya B, Ukwa UD, Odeozie J, Ganiu Y (2014) Host condition, parasite interaction and metal accumulation in Tilapia guineensis from Iddo area of Lagos lagoon, Nigeria. Iran J Ichthyol 1:289–297
Scheef G, Sures B, Taraschewski H (2000) Cadmium accumulation in Moniliformis moniliformis (Acanthocephala) from experimentally infected rats. Parasitol Res 86:688–691. doi:10.1007/PL00008553
Schintu M, Durante L, Maccioni A, Meloni P, Degetto S, Contu A (2008) Measurement of environmental trace-metal levels in Mediterranean coastal areas with transplanted mussels and DGT techniques. Mar Pollut Bull 57:832–837. doi:10.1016/j.marpolbul.2008.02.038
Schludermann C, Konecny R, Laimgruber S, Lewis JW, Schiemer F, Chovanec A, Sures B (2003) Fish macroparasites as indicators of heavy metal pollution in river sites in Austria. Parasitology 126(Suppl):S61–S69. doi:10.1017/S0031182003003743
Schmidt V, Zander S, Körting W, Steinhagen D (2003) Parasites of the flounder Platichthys flesus (L.) from the German Bight, North Sea, and their potential use in ecosystem monitoring: A. Infection characteristics of potential indicator species. Helgol Mar Res 57:236–251. doi:10.1007/s10152-003-0147-1
Šebelová Š, Kuperman B, Gelnar M (2002) Abnormalities of the attachment clamps of representatives of the family Diplozoidae. J Helminthol 76:249–259. doi:10.1079/JOH2002133
Shah HB, Yousuf AR, Chishti MZ, Ahmad F (2013) Helminth communities of fish as ecological indicators of lake health. Parasitology 140:352–360. doi:10.1017/S0031182012001679
Shinn AP, Gibson DI, Sommerville C (1995) A study of the composition of the sclerites of Gyrodactylus Nordmann, 1832 (Monogenea) using X-ray elemental analysis. Int J Parasitol 25:797–805. doi:10.1016/0020-7519(95)00008-P
Shukla AK, Patra S, Dubey V (2012) Iridoid glucosides from Nyctanthes arbortristis result in increased reactive oxygen species and cellular redox homeostasis imbalance in Leishmania parasite. Eur J Med Chem 54:49–58. doi:10.1016/j.ejmech.2012.04.034
Siddall R, Sures B (1998) Uptake of lead by Pomphorhynchus laevis cystacanths in Gammarus pulex and immature worms in chub (Leuciscus cephalus). Parasitol Res 84:573–577. doi:10.1007/s004360050451
Siddall R, Koskivaara M, Valtonen ET (1997) Dactylogyrus (Monogenea) infections on the gills of roach (Rutilus rutilus L.) experimentally exposed to pulp and paper mill effluent. Parasitology 114:439–446. doi:10.1017/S003118209600875X
Siegel BZ, Siegel SM, Correa T, Dagan C, Galvez G, LeeLoy L, Padua A, Yaeger E (1991) The protection of invertebrates, fish, and vascular plants against inorganic mercury poisoning by sulfur and selenium derivatives. Arch Environ Contam Toxicol 20:241–246. doi:10.1007/BF01055910
Šimková A, Sasal P, Kadlec D, Gelnar M (2001) Water temperature influencing dactylogyrid species communities in roach, Rutilus rutilus, in the Czech Republic. J Helminthol 75:373–383. doi:10.1079/JOH200176
Skinner RH (1982) The interrelation of water quality, gill parasites, and gill pathology of some fishes from South Biscayne Bay, Florida. Fish Bull 80:269–280
Smith GL, Baker TR (2003) Lichens as bioindicators. Sci Scope 27:16–19
Soleng A, Bakke TA (1997) Salinity tolerance of Gyrodactylus salaris (Platyhelminthes, Monogenea): laboratory studies. Can J Fish Aquat Sci 54:1837–1845
Soleng A, Bakke TA, Hansen LP (1998) Potential for dispersal of Gyrodactylus salaris (Platyhelminthes, Monogenea) by sea-running stages of the Atlantic salmon (Salmo salar): field and laboratory studies. Can J Fish Aquat Sci 55:507–514. doi:10.1139/cjfas-55-2-507
Soleng A, Poléo ABS, Alstad NEW, Bakke TA (1999) Aqueous aluminium eliminates Gyrodactylus salaris (Platyhelminthes, Monogenea) infections in Atlantic salmon. Parasitology 119:19–25. doi:10.1017/S0031182099004436
Soleng A, Poléo ABS, Bakke TA (2005) Toxicity of aqueous aluminium to the ectoparasitic monogenean Gyrodactylus salaris. Aquaculture 250:616–620. doi:10.1016/j.aquaculture.2005.05.006
Squadrone S, Prearo M, Brizio P, Gavinelli S, Pellegrino M, Scanzio T, Guarise S, Benedetto A, Abete MC (2013) Heavy metals distribution in muscle, liver, kidney and gill of European catfish (Silurus glanis) from Italian rivers. Chemosphere 90:358–365. doi:10.1016/j.chemosphere.2012.07.028
Sures B (2001) The use of fish parasites as bioindicators of heavy metals in aquatic ecosystems: a review. Aquat Ecol 35:245–255. doi:10.1023/A:1011422310314
Sures B (2003) Accumulation of heavy metals by intestinal helminths in fish: an overview and perspective. Parasitology 126:53–60. doi:10.1017/S003118200300372X
Sures B (2004) Environmental parasitology: relevancy of parasites in monitoring environmental pollution. Trends Parasitol 20:170–177. doi:10.1016/j.pt.2004.01.014
Sures B (2005) Effects of pollution on parasites and use of parasites in pollution monitoring. In: Rohde K (ed) Marine parasitology. CABI Publishing, London, pp 421–425
Sures B (2006) How parasitism and pollution affect the physiological homeostasis of aquatic hosts. J Helminthol 80:151–157. doi:10.1079/JOH2006346
Sures B (2008) Host-parasite interactions in polluted environments. J Fish Biol 73:2133–2142. doi:10.1111/j.1095-8649.2008.02057.x
Sures B, Radszuweit H (2007) Pollution-induced heat shock protein expression in the amphipod Gammarus roeseli is affected by larvae of Polymorphus minutus (Acanthocephala). J Helminthol 81:191–197. doi:10.1017/S0022149X07751465
Sures B, Reimann N (2003) Analysis of trace metals in the Antarctic host-parasite system Notothenia coriiceps and Aspersentis megarhynchus (Acanthocephala) caught at King George Island, South Shetland Islands. Polar Biol 26:680–686. doi:10.1007/s00300-003-0538-4
Sures B, Siddall R (1999) Pomphorhynchus laevis: the intestinal acanthocephalan as a lead sink for its fish host, chub (Leuciscus cephalus). Exp Parasitol 93:66–72. doi:10.1006/expr.1999.4437
Sures B, Siddall R (2001) Comparison between lead accumulation of Pomphorhynchus laevis (Palaeacanthocephala) in the intestine of chub (Leuciscus cephalus) and in the body cavity of goldfish (Carassius auratus auratus). Int J Parasitol 31:669–673
Sures B, Siddall R (2003) Pomphorhynchus laevis (Palaeacanthocephala) in the intestine of chub (Leusciscus cephalus) as an indicator of metal pollution. Int J Parasitol 33:65–70. doi:10.1016/S0020-7519(02)00249-7
Sures B, Taraschewski H (1995) Cadmium concentrations in two adult acanthocephalans, Pomphorhynchus laevis and Acanthocephalus lucii, as compared with their fish hosts and cadmium and lead levels in larvae of A. lucii as compared with their crustacean host. Parasitol Res 81:494–497. doi:10.1007/BF00931792
Sures B, Taraschewski H, Jackwerth E (1994a) Lead content of Paratenuisentis ambiguus (Acanthocephala), Anguillicola crassus (Nematodes) and their host Anguilla anguilla. Dis Aquat Org 19:105–107
Sures B, Taraschewski H, Jackwerth E (1994b) Comparative study of lead accumulation in different organs of perch (Perca fluviatilis) and its intestinal parasite Acanthocephalus lucii. Bull Environ Contam Toxicol 52:269–273. doi:10.1007/BF00198498
Sures B, Taraschewski H, Jackwerth E (1994c) Lead accumulation in Pomphorhynchus laevis and its host. J Parasitol 80:355–357
Sures B, Taraschewski H, Rokicki J (1997a) Lead and cadmium content of two cestodes, Monobothrium wageneri and Bothriocephalus scorpii and their fish hosts. Parasitol Res 83:618–623. doi:10.1007/s004360050307
Sures B, Taraschewski H, Rydlo M (1997b) Intestinal fish parasites as heavy metal bioindicators: a comparison between Acanthocephalus lucii (Palaeacanthocephala) and the zebra mussel, Dreissena polymorpha. Bull Environ Contam Toxicol 59:14–21. doi:10.1007/s001289900437
Sures B, Jürges G, Taraschewski H (1998) Relative concentrations of heavy metals in the parasites Ascaris suum (Nematoda) and Fasciola hepatica (Digenea) and their respective porcine and bovine definitive hosts. Int J Parasitol 28:1173–1178. doi:10.1016/S0020-7519(98)00105-2
Sures B, Siddall R, Taraschewski H (1999) Parasites as accumulation indicators of heavy metal pollution. Parasitol Today 15:16–21. doi:10.1016/S0169-4758(98)01358-1
Sures B, Grube K, Taraschewski H (2002) Experimental studies on the lead accumulation in the cestode Hymenolepis diminuta and its final host, Rattus norvegicus. Ecotoxicology 11:365–368. doi:10.1023/A:1020561406624
Sures B, Scheible T, Bashtar AR, Taraschewski H (2003a) Lead concentrations in Hymenolepis diminuta adults and Taenia taeniaeformis larvae compared to their rat hosts (Rattus norvegicus) sampled from the city of Cairo, Egypt. Parasitology 127:483–487. doi:10.1017/S0031182003003901
Sures B, Dezfuli BS, Krug HF (2003b) The intestinal parasite Pomphorhynchus laevis (Acanthocephala) interferes with the uptake and accumulation of lead (210Pb) in its fish host chub (Leuciscus cephalus). Int J Parasitol 33:1617–1622. doi:10.1016/S0020-7519(03)00251-0
Sures B, Thielen F, Baska F, Messerschmidt J, von Bohlen A (2005) The intestinal parasite Pomphorhynchus laevis as a sensitive accumulation indicator for the platinum group metals Pt, Pd, and Rh. Environ Res 98:83–88. doi:10.1016/j.envres.2004.05.010
Sures B, Nachev M, Selbach C, Marcogliese DJ (2017) Parasite responses to pollution: what we know and where we go in “environmental parasitology”. Parasit Vectors 10:65. doi:10.1186/s13071-017-2001-3
Szefer P, Rokicki J, Frelek K, Skóra K, Malinga M (1998) Bioaccumulation of selected trace elements in lung nematodes, Pseudalius inflexus, of harbor porpoise (Phocoena phocoena) in a Polish zone of the Baltic Sea. Sci Total Environ 220:19–24
Tao S, Liang T, Cao J, Dawson RW, Liu C (1999) Synergistic effect of copper and lead uptake by fish. Ecotoxicol Environ Saf 44:190–195. doi:10.1006/eesa.1999.1822
Tate RB, Husted A (2015) Aquatic macroinvertebrate responses to pollution of the Boesmanspruit River system above Carolina, South Africa. Afr J Aquat Sci 40:153–163. doi:10.2989/16085914.2015.1037237
Taylor JC, Prygiel J, Vosloo A, de la Rey PA, van Rensberg L (2007) Can diatom-based pollution indices be used for biomonitoring in South Africa? A case study of the Crocodile West and Marico water management area. Hydrobiologia 592:455–464. doi:10.1007/s10750-007-0788-1
Tekin-Özan S, Barlas M (2008) Concentrations of selected heavy metals in Ligula intestinalis L., 1758 plerocercoids (Cestoda) compared to it host’s (Tinca tinca L., 1758) organs from Beyşehir Lake (Turkey). Helminthologia 45:76–80. doi:10.2478/s11687-008-0014-3
Tekin-Özan S, Kir I (2005) Comparative study on the accumulation of heavy metals in different organs of tench (Tinca tinca L. 1758) and plerocercoids of its endoparasite Ligula intestinalis. Parasitol Res 97:156–159. doi:10.1007/s00436-005-1412-9
Tellez M, Merchant M (2015) Biomonitoring heavy metal pollution using an aquatic apex predator, the American alligator, and its parasites. PLoS One 10:1–18. doi:10.1371/journal.pone.0142522
Thielen F, Zimmermann S, Baska F, Taraschewski H, Sures B (2004) The intestinal parasite Pomphorhynchus laevis (Acanthocephala) from barbel as a bioindicator for metal pollution in the Danube River near Budapest, Hungary. Environ Pollut 129:421–429. doi:10.1016/j.envpol.2003.11.011
Thoney DA (1990) The effects of trichlorfon, praziquantel and copper sulphate on various stages of the monogenean Benedeniella posterocolpa, a skin parasite of the cownose ray, Rhinoptera bonasus (Mitchill). J Fish Dis 13:385–389. doi:10.1111/j.1365-2761.1990.tb00797.x
Tóro RM, Gessner AAF, Furtado NAJC, Ceccarelli PS, de Albuquerque S, Bastos JK (2003) Activity of the Pinus elliottii resin compounds against Lernaea cyprinacea in vitro. Vet Parasitol 118:143–149
Torres J, Peig J, Eira C, Borrás M (2006) Cadmium and lead concentrations in Skrjabinotaenia lobata (Cestoda: Catenotaeniidae) and in its host, Apodemus sylvaticus (Rodentia: Muridae) in the urban dumping site of Garraf (Spain). Environ Pollut 143:4–8. doi:10.1016/j.envpol.2005.11.012
Torres J, Kacem H, Eira C, Neifar L, Miquel J (2014) Total mercury and selenium concentrations in Sarpa salpa and Balistes capriscus and in their respective digenean endoparasites Robphildollfusium fractum and Neoapocreadium chabaudi from Tunisia. Acta Parasitol 59:580–585. doi:10.2478/s11686-014-0293-4
Tsotetsi AM, Avenant-Oldewage A, Mashego SN (2004) Aspects of the ecology of Lamproglena clariae ( Copepoda: Lernaeidae ) from the Vaal River system, South Africa. J Crustac Biol 24:529–536
Tuuha H, Valtonen ET, Taskinen J (1992) Ergasilid copepods as parasites of perch Perca fluviatilis and roach Rutilus rutilus in central Finland: seasonality, maturity and environmental influence. J Zool 228:405–422
Valtonen ET, Holmes JC, Koskivaara M (1997) Eutrophication, pollution and fragmentation: effects on parasite communities in roach (Rutilus rutilus) and perch (Perca fluviatilis) in four lakes in Central Finland. Can J Fish Aq Sci 54:572–585. doi:10.1139/cjfas-54-3-572
Valtonen ET, Holmes JC, Aronen J, Rautalahti I (2003) Parasite communities as indicators of recovery from pollution: parasites of roach (Rutilus rutilus) and perch (Perca fuviatilis) in central Finland. Parasitology 126(Suppl):S43–S52. doi:10.1017/S0031182003003494
Van der Schyff V, Pieters R, Bouwman H (2016) The heron that laid the golden egg: metals and metalloids in ibis, darter, cormorant, heron, and egret eggs from the Vaal River catchment, South Africa. Environ Monit Assess 188:372. doi:10.1007/s10661-016-5378-0
Vidal-Martínez VM, Pech D, Sures B, Purucker ST, Poulin R (2010) Can parasites really reveal environmental impact? Trends Parasitol 26:44–51. doi:10.1016/j.pt.2009.11.001
Vidal-Martínez VM, Centeno-Chalé OA, Torres-Irineo E, Sánchez-Ávila J, Gold-Bouchot G, Aguirre-Macedo ML (2014) The metazoan parasite communities of the shoal flounder (Syacium gunteri) as bioindicators of chemical contamination in the southern Gulf of Mexico. Parasit Vectors 7:541. doi:10.1186/s13071-014-0541-3
Wah Chu K, Chow KL (2002) Synergistic toxicity of multiple heavy metals is revealed by a biological assay using a nematode and its transgenic derivative. Aquat Toxicol 61:53–64. doi:10.1016/S0166-445X(02)00017-6
Wang W (1987) Factors affecting metal toxicity to (and accumulation by) aquatic organisms—overview. Environ Int 13:437–457
Watson RM, Crafford D, Avenant-Oldewage A (2012) Evaluation of the fish health assessment index in the Olifants River system, South Africa. Afr J Aquat Sci:1–17. doi:10.2989/16085914.2012.677745
Wei K, Yang J (2015) Oxidative damage induced by copper and beta-cypermethrin in gill of the freshwater crayfish Procambarus clarkii. Ecotoxicol Environ Saf 113:446–453. doi:10.1016/j.ecoenv.2014.12.032
Wepener V, van Vuren JH, Chatiza FP, Mbizi Z, Slabbert L, Masola B (2005) Active biomonitoring in freshwater environments: early warning signals from biomarkers in assessing biological effects of diffuse sources of pollutants. Phys Chem Earth 30:751–761. doi:10.1016/j.pce.2005.08.018
Wepener V, van Dyk C, Bervoets L, O’Brien G, Covaci A, Cloete Y (2011) An assessment of the influence of multiple stressors on the Vaal River, South Africa. Phys Chem Earth 36:949–962. doi:10.1016/j.pce.2011.07.075
Weston LF, Reed C, Hendricks M, Winker H, Van der Lingen CD (2015) Stock discrimination of South African sardine (Sardinops sagax) using a digenean parasite biological tag. Fish Res 164:120–129. doi:10.1016/j.fishres.2014.11.002
Williams HH, Mackenzie K (2003) Marine parasites as pollution indicators: an update. Parasitology 126:27–41. doi:10.1017/S0031182003003640
Woelfl S, Mages M, Torres P (2008) Trace metal concentrations in single specimens of the intestinal broad flatworm (Diphyllobothrium latum), compared to their fish host (Oncorhynchus mykiss) measured by total reflection X-ray fluorescence spectrometry. Spectrochim Acta - Part B At Spectrosc 63:1450–1454. doi:10.1016/j.sab.2008.10.009
Yen Nhi TT, Mohd Shazili NA, Shaharom-Harrison F (2013) Use of cestodes as indicator of heavy-metal pollution. Exp Parasitol 133:75–79. doi:10.1016/j.exppara.2012.10.014
Yeomans WE, Chubb JC, Sweeting RA (1997) Use of protozoan communities for pollution monitoring. Parassitologia 39:201–212
Zander CD, Reimer LW (2002) Parasitism at the ecosystem level in the Baltic Sea. Parasitology 124:119–135. doi:10.1017/S0031182002001567
Zargar UR, Chishti MZ, Yousuf AR, Fayaz A (2012a) Infection level of monogenean gill parasite, Diplozoon kashmirensis (Monogenea, Polyopisthocotylea) in the Crucian carp, Carassius carassius from lake ecosystems of an altered water quality: what factors do have an impact on the Diplozoon infection? Vet Parasitol 189:218–226. doi:10.1016/j.vetpar.2012.04.029
Zargar UR, Yousuf AR, Chishti MZ, Ahmed F, Bashir H, Ahmed F (2012b) Effects of water quality and trophic status on helminth infections in the cyprinid fish, Schizothorax niger Heckel, 1838 from three lakes in the Kashmir Himalayas. J Helminthol 86:70–76. doi:10.1017/S0022149X11000071
Zharikova TI (1993) Effect of water pollution on ectoparasites of bream (Abramis brama). J Ichthyol 33:50–62
Zhou Q, Zhang J, Fu J, Shi J, Jiang G (2008) Biomonitoring: an appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal Chim Acta 606:135–150. doi:10.1016/j.aca.2007.11.018
Zimmermann S, Sures B, Taraschewski H (1999) Experimental studies on lead accumulation in the eel-specific endoparasites Anguillicola crassus (Nematoda) and Paratenuisentis ambiguus (Acanthocephala) as compared with their host, Anguilla anguilla. Arch Environ Contam Toxicol 37:190–195. doi:10.1007/s002449900505
Zimmermann S, von Bohlen A, Messerschmidt J, Sures B (2005) Accumulation of the precious metals platinum, palladium and rhodium from automobile catalytic converters in Paratenuisentis ambiguus as compared with its fish host, Anguilla anguilla. J Helminthol 79:85–89. doi:10.1079/JOH2004261
Acknowledgements
The authors extend thanks to the National Research Foundation and the University of Johannesburg for providing funding to AAO.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Gilbert, B.M., Avenant-Oldewage, A. Parasites and pollution: the effectiveness of tiny organisms in assessing the quality of aquatic ecosystems, with a focus on Africa. Environ Sci Pollut Res 24, 18742–18769 (2017). https://doi.org/10.1007/s11356-017-9481-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9481-8