Abstract
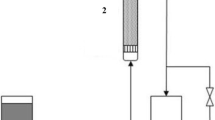
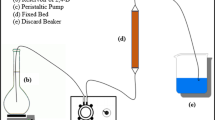
The adsorption of o-xylene onto raw clay material in a fixed bed using a thermal conductivity detector gas chromatography was investigated. Experimental and theoretical studies were established to evaluate the removal efficiency of o-xylene by adsorption on clay materials and to predict kinetic parameters. Column data were describing at different conditions using Bohart–Adams, Wolborska, Thomas, Yoon and Nelson, dose–response, and bed depth service time models. All used models were satisfactory to predict the breakthrough curves. A suitable advection–dispersion–sorption (ADS) model has been also developed to simulate the measured data, based on the nature of the various equilibrium relationships of solid–gas and diverse descriptions of the mass transfer processes within of the adsorbent particle. The experiments can be fitted with high correlated coefficient R 2 = 0.996.







Similar content being viewed by others
Abbreviations
- a :
-
Dose–response constant
- a p :
-
Specific and volumetric surface for spherical particle (m−1)
- C 0 :
-
Inlet o-xylene concentration (g m−3)
- C :
-
Outlet concentration of the solute in the fluid phase at time t (g m−3)
- C i :
-
Concentration of solute in the solid–gas interface (g m−3)
- D eff :
-
Effective coefficient diffusion (m2 min−1)
- D ax :
-
The axial dispersion coefficient (m2 min−1)
- d p :
-
Particle diameter (m)
- K BA :
-
Kinetic constant of Bohat–Adams model (m3 g−1 min−1)
- K BDST :
-
Kinetic constant of BDST model (m3 g−1 min−1)
- K C :
-
Kinetic constant of Clark (m3 g−1 min−1)
- k f :
-
Mass transfer coefficient in the fluid phase (m s−1)
- K L :
-
Langmuir constant
- k s :
-
Mass transfer coefficient in solid phase (m s−1)
- K YN :
-
Kinetic constant of Yoon–Nelson model (min−1)
- K Th :
-
Kinetic constant of Thomas model (m3 g−1 min−1)
- L c :
-
Column length (mm)
- m :
-
Amount of adsorbent in the column (g)
- n :
-
Freundlich constant
- N 0 :
-
Maximum volumetric sorption capacity of bed (g m−3)
- Q :
-
Flow rate (m3 min−1)
- q 0 :
-
Maximum adsorption capacity (mg g−1)
- q :
-
Average weight of the solute adsorbed at time t, per kg of fresh adsorbent (mg g−1)
- q i :
-
The weight of the solute adsorbed at time t, per kg of fresh adsorbent solid–gas interface (mg g−1)
- S :
-
Cross section of the column (m2)
- t :
-
Time (min)
- u :
-
Linear velocity (m min−1)
- v :
-
Interstitial fluid velocity (m min−1); (\( \mathit{\mathsf{v}}=\frac{\mathit{\mathsf{u}}}{\varepsilon} \))
- Z :
-
Bed height (m)
- β a :
-
The kinetic coefficient of the external mass transfer (min−1)
- ρ :
-
Density of the bed (g m−3)
- ε :
-
Void fraction of the bed
References
Acheampong MA, Pakshirajan K, Annachhatre AP, Lens PNL (2013) Removal of Cu (II) by biosorption onto coconut shell in fixed-bed column systems. J Ind Eng Chem 19:841–848
Afzal S, Rahimi A, Ehsani MR, Tavakoli H (2010) Modeling hydrogen fluoride adsorption bysodium fluoride. J Ind Eng Chem 16:978–985
Akhnazarova S, Kafarov V (1982) Experiment optimization in chemistry and chemical engineering. Mir Publishers, Moscow
Aksu Z, Gönen F (2006) Binary biosorption of phenol and chromium (VI) onto immobilized activated sludge in a packed bed: prediction of kinetic parameters and breakthrough curves. Sep Purif Technol 49:205–216
Amari A, Gannouni A, Chlendi M, Bellagi A (2008) Optimization by response surface methodology (RSM) for toluene adsorption onto prepared acid activated clay. Can J Chem Eng 86:1093–1102
Bohart GS, Adams EQ (1920) Some aspects of the behaviour of the charcoal with respect chlorine. J Am Chem Soc 42:523–544
Calero de Hoces M, Blázquez García G, Ronda Gálvez A, Martín-Lara MA (2010) Effect of the acid treatment of olive stone on the biosorption of lead in a packed-bed column. Ind Eng Chem Res 49:12587–12595
Calero M, Hernáinz F, Blázquez G, Tenorio G, Martín-Lara MA (2009) Study of Cr (III) biosorption in a fixed-bed column. J Hazard Mater 171:886–893
Chafik T, Harti S, Cifredo G, Gatica JM, Vidal H (2009) Easy extrusion of honeycomb-shaped monoliths using Moroccan natural clays and investigation of their dynamic adsorptive behavior towards VOCs. J Hazard Mater 170:87–95
Chen N, Zhang Z, Feng C, Li M, Chen R, Sugiura N (2011) Investigations on the batch and fixed-bed column performance of fluoride adsorption by Kanuma mud. Desalination 268:76–82
Chu KH (2004) Improved fixed bed models for metal biosorption. Chem Eng J 97(2–3):233–239
Clark RM (1987) Evaluating the cost and performance of field-scale granular activated carbon systems. Environ Sci Technol 21:573–580
Cloirec PL (2003) Adsorption en traitement de l’air. Techniques de l’ingénieur G1 770:13
Dammak N, Fakhfakh N, Fourmentin S, Benzina M (2013) Natural clay as raw and modified material for efficient o-xylene abatement. J Env Chem Eng 1:667–675
Dammak N, Fakhfakh N, Fourmentin S, Benzina M (2015) Treatment of gas containing hydrophobic VOCs by adsorption process on raw and intercalated clays. Res Chem Intermed 41:5475–5493
Dammak N, Ouledltaif O, Fakhfakh N, Benzina M (2014) Adsorption equilibrium studies for oxylene vapour and modified clays system. Surf Interface Anal 46:457–464
Edward MF, Richardson JF (1968) Gas dispersion in packed beds. Chem Eng Sci 23:109–123
Guibal E, Lorenzelli R, Vincent T, Cloirec L (1995) Application of silica gel to metal ion sorption: static and dynamic removal of uranyl ions. Environ Technol 16:101–114
Gupta VK, Verma N (2002) Removal of volatile organic compounds by cryogenic condensation followed by adsorption. Chem Eng Sci 57:2679–2696
Haaland PD (1989) Separating signals from the noise in experimental design in biotechnology. Marcel Dekker, New York, pp 61–83
Hamdaoui O (2006) Dynamic sorption of methylene blue by cedar sawdust and crushed brick in fixed bed columns. J Hazard Mater 138:293–303
Han R, Ding D, Xu Y, Zou W, Wang Y, Li Y, Zou L (2008) Use of rice husk for the adsorption of congo red from aqueous solution in column mode. Bioresour Technol 99:2938–2946
Han RP, Zou LN, Zhao X, Xu YF, Xu F, Li YL, Wang Y (2009) Characterization and properties of iron oxide-coated zeolite as adsorbent for removal of copper (II) from solution in fixed bed column. Chem Eng J 149:123–131
Hasan SH, Ranjan D, Talat M (2010) Agro-industrial waste ‘wheat bran’ for the biosorptive remediation of selenium through continuous up-flow fixed-bed column. J Hazard Mater 181:1134–1142
Ho CK, Webb SW (eds) (2006) Gas transport in porous media. Springer, Dordrecht
Huang S, Zhang C, He H (2009) In situ adsorption-catalysis system for the removal of o-xylene over an activated carbon supported Pd catalyst. J Environ Sci 21:985–990
Huang ZH, Kang F, Liang KM, Hao J (2003) Breakthrough of methyethylketone and benzene vapors in activated carbon fiber beds. J Hazard Mater 98:107–115
Jones AP (1999) Indoor air quality and health. Atmos Environ 33(28):4535–4564
Khuri AI, Cornell JA (1996) Response surfaces: design and analysis, 2nd edn. Marcel Dekker, New York
Ko DCK, Porter JF, McKay G (2000) Optimised correlations for the fixed bed adsorption of metal ions on bone char. Chem Eng Sci 55:5819–5829
Kumar PA, Chakraborty S (2009) Fixed-bed column study for hexavalent chromium removal and recovery by short-chain polyaniline synthesized on jute fiber. J Hazard Mater 162:1086–1098
Lee JF, Mortland MM, Chiou CT, Kile DE, Boyd SA (1990) Adsorption of benzene, toluene, and xylene by two tetramethylammonium-smectites having different charge densities. Clay Clay Miner 38(2):113–120
Leinekugel-le-Cocq D, Tayakout-Fayolle M, Le Gorrec Y, Jallut C (2007) A double linear driving force approximation for non-isothermal mass transfer modeling through bi-disperse adsorbents. Chem Eng Sci 62:4040–4053
Leyva-Ramos R, Diaz-Flores PE, Leyva-Ramos J, Femat-Flores RA (2007) Kinetic modeling of pentachlorophenol adsorption from aqueous solution on activated carbon fibers. Carbon 45:2280–2289
Lin SH, Cheng MJ (2002) Adsorption of phenol and m-chlorophenol on organo bentonites and repeated thermal regeneration. Waste Manag 22:595–603
Lua AC, Jandia QP (2009) Adsorption of phenol by oil-palm-shell activated carbons in a fixed bed. Chem Eng J 150:455–461
Malek A, Farooq S (1997) Kinetics of hydrocarbon adsorption on activated carbon and silica gel. AICHE j 43:761–776
Minceva M, Rodrigues AE (2004) Adsorption of xylenes on faujasite-type zeolite equilibrium and kinetics in batch adsorber. Chem Eng Res and Des 82(A5):667–681
Morales Futulan C, Kan CC, Dalida ML, Pascua C, Wan WW (2011) Fixed-bed column studies on the removal of copper using chitosan immobilized on bentonite. Carbohydr Polym 83:697–704
Muhamad H, Doan H, Lohi A (2010) Batch and continuous fixed-bed column biosorption of Cd2+ and Cu2+. Chem Eng J 158:369–377
Pires J, Carvalho A, De Carvalho MB (2001) Adsorption of volatile organic compounds in Y zeolites and pillared clays. Micorpor Mesopor Mater 43:277–287
Qaiser S, Saleemi AR, Umar M (2009) Biosorption of lead from aqueous solution by Ficusreligiosa leaves: batch and column study. J Hazard Mater 166:998–1005
Rao KS, Anad S, Venkateswarlu P (2011) Modeling the kinetics of Cd(II) adsorption on Syzygiumcumini L. leaf powder in a fixed bed mini column. J Ind Eng Chem 17:174–181
Rutherford SW, Do DD (2000a) Adsorption dynamics measured by permeation and batch adsorption methods. Chem Eng J 76:23–31
Rutherford SW, Do DD (2000b) Adsorption dynamics of carbon dioxide on a carbon molecular sieve 5A. Carbon 38:1339–1350
Sarin V, Singh TS, Pant KK (2006) Thermodynamic and breakthrough column studies for the selective sorption of chromium from industrial effluent on activated eucalyptus bark. Bioresour Technol 97:1986–1993
Simo M, Brown CJ, Hlavacek V (2008) Simulation of pressure swing adsorption in fuel ethanol production process. Comput Chem Eng 32:1635–1649
Thomas HC (1944) Heterogeneous ion exchange in a flowing system. J Am Chem Soc 66:1664–1666
Tor A, Danaoglu N, Arslan G, Cengeloglu Y (2009) Removal of fluoride from water by using granular red mud: batch and column studies. J Hazard Mater 164:271–278
Vijayaraghavan K, Prabu D (2006) Potential of Sargassum wightii biomass for copper (II) removal from aqueous solutions: application of the different mathematical models to batch and continuous biosorption data. J Hazard Mater 137:558–564
Vinodhini V, Das N (2010) Packed bed column studies on Cr (VI) removal from tannery wastewater by neem sawdust. Desalination 264:9–14
Wolborska A (1989) Adsorption on activated carbon of p-nitrophenol from aqueous solution. Water Res 23:85–91
Yan GY, Viraraghavan T, Chem M (2001) A new model for heavy metal removal in a biosorption column. Adsorpt Sci Technol 19:25–43
Yaneva Z, Marinkovski M, Markovska L, Meshko V, Koumanova B (2008) Dynamic studies of nitrophenols sorption on perfil in a fixed-bed column. Maced J Chem Chem Eng 27:123–132
Yoon YH, Nelson JH (1984) Application of gas adsorption kinetics. I. A theoretical model for respirator cartridge service life. Am Ind Hyg Assoc J 45:509–516
Zhang X, Chen S, Bi HT (2010) Application of wave propagation theory to adsorption breakthrough studies of toluene on activated carbon fiber beds. Carbon 48:2317–2326
Acknowledgements
The authors are grateful to PHC Maghreb no. 27959PD for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Marcus Schulz
Rights and permissions
About this article
Cite this article
Fakhfakh, N., Dammak, N. & Benzina, M. Breakthrough modeling and experimental design for o-xylene dynamic adsorption onto clay material. Environ Sci Pollut Res 25, 18263–18277 (2018). https://doi.org/10.1007/s11356-017-9386-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9386-6