Abstract
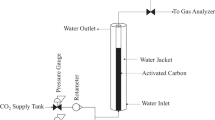
The performance of an adsorption column packed with granular activated carbon was evaluated for the removal of phenols from refinery wastewater. The effects of phenol feed concentration (80–182 mg/l), feed flow rate (5–20 ml/min), and activated carbon packing mass (5–15 g) on the breakthrough characteristics of the adsorption system were determined. The continuous adsorption process was simulated using batch data and the parameters for a new empirical model were determined. Different dynamic models such as Adams–Bohart, Wolborsko, Thomas, and Yoon-Nelson models were also fitted to the experimental data for the sake of comparison. The empirical, Yoon–Nelson and Thomas models showed a high degree of fitting at different operation conditions, with the empirical model giving the best fit based on the Akaike information criterion (AIC). At an initial phenol concentration of 175 mg/l, packing mass of 10 g, a flow rate of 10 ml/min and a temperature of 25 °C, the SSE of the new empirical and Thomas models were identical (248.35) and very close to that of the Yoon–Nelson model (259.49). The values were significantly lower than that of the Adams–Bohart model, which was determined to be 19,358.48. The superiority of the new empirical model and the Thomas model was also confirmed from the values of the R 2 and AIC, which were 0.99 and 38.3, respectively, compared to 0.92 and 86.2 for Adams–Bohart model.








Similar content being viewed by others
References
Abdelwahab O, Amin NK, El-Ashtoukhy ESZ (2009) Electrochemical removal of phenol from oil refinery wastewater. J Hazard Mater 163(2–3):711–716
Ahmad AA, Hameed BH (2010) Fixed-bed adsorption of reactive azo dye onto granular activated carbon prepared from waste. J Hazard Mater 175(1–3):298–303
Akaike, H. (1973) Information theory as an extension of the maximum likelihood principle, Second International Symposium on Information Theory, Budapest, Hungary: Akademiai Kiado, 267–281.
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19(6):716–723
Akpa OM, Unuabonah EI (2011) Small-sample corrected Akaike information criterion: an appropriate statistical tool for ranking of adsorption isotherm models. Desalination 272(1–3):20–26
Aksu Z, Gönen F, Demircan Z (2002) Biosorption of chromium(VI) ions by Mowital®B30H resin immobilized activated sludge in a packed bed: comparison with granular activated carbon. Process Biochem 38(2):175–186
Al Zarooni M, Elshorbagy W (2006) Characterization and assessment of Al Ruwais refinery wastewater. J Hazard Mater 136(3):398–405
Alhamed YA (2009) Adsorption kinetics and performance of packed bed adsorber for phenol removal using activated carbon from dates’ stones. J Hazard Mater 170(2–3):763–770
Bohart GS, Adams EQ (1920) Some aspects of the behavior of charcoal with respect to chlorine. J Am Chem Soc 42:523–529
Burnham, K. P. and Anderson, D. R. (2002) Model selection and multimodel inference : a practical information-theoretic approach. 2nd edn. New York: Springer.
Chu KH (2010) Fixed bed sorption: setting the record straight on the Bohart–Adams and Thomas models. J Hazard Mater 177(1–3):1006–1012
El-Khaiary MI, Malash GF (2011) Common data analysis errors in batch adsorption studies. Hydrometallurgy 105(3–4):314–320
El-Naas MH, Makhlouf S (2008) A spouted bed bioreactor for the biodegradation of phenols in refinery wastewater. J Biotechnol 136(Supplement 1):S650
El-Naas MH, Al-Zuhair S, Alhaija MA (2010a) Reduction of COD in refinery wastewater through adsorption on date-pit activated carbon. J Hazard Mater 173(1–3):750–757
El-Naas MH, Al-Zuhair S, Alhaija MA (2010b) Removal of phenol from petroleum refinery wastewater through adsorption on date-pit activated carbon. Chem Eng J 162(3):997–1005
El-Naas MH, Al-Zuhair S, Makhlouf S (2010c) Continuous biodegradation of phenol in a spouted bed bioreactor (SBBR). Chem Eng J 160(2):565–570
Girods P, Dufour A, Fierro V, Rogaume Y, Rogaume C, Zoulalian A, Celzard A (2009) Activated carbons prepared from wood particleboard wastes: characterisation and phenol adsorption capacities. J Hazard Mater 166(1):491–501
Hameed BH, Rahman AA (2008) Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. J Hazard Mater 160(2–3):576–581
Lin S-H, Juang R-S (2009) Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: a review. J Environ Manag 90(3):1336–1349
Lin SH, Wang CS (2002) Treatment of high-strength phenolic wastewater by a new two-step method. J Hazard Mater 90(2):205–216
Ma H, Zhang X, Ma Q, Wang B (2009) Electrochemical catalytic treatment of phenol wastewater. J Hazard Mater 165(1–3):475–480
Rajkumar. D, K. Palanivelu, and N. Balasubramanian 2005. Combined electrochemical degradation and activated carbon adsorption treatments for wastewater containing mixed phenolic compounds.
Richard D, De Lourdes Delgado-Nunez M, Schweich D (2010) Adsorption of complex phenolic compounds on active charcoal: breakthrough curves. Chem Eng J 158(2):213–219
Shirgaonkar IZ, Pandit AB (1998) Sonophotochemical destruction of aqueous solution of 2,4,6-trichlorophenol. Ultrason Sonochem 5(2):53–61
Texier AC, Andrès Y, Faur-Brasquet C, Le Cloirec P (2002) Fixed-bed study for lanthanide (La, Eu, Yb) ions removal from aqueous solutions by immobilized Pseudomonas Aeruginosa: experimental data and modelization. Chemosphere 47(3):333–342
Thomas HC (1944) Hetergeneous ion exchange in a flowing system. J Am Chem Soc 66:1466–1664
Trivedi U, Bassi A, Zhu J-X (2006) Continuous enzymatic polymerization of phenol in a liquid-solid circulating fluidized bed. Powder Technol 169(2):61–70
Ugurlu M, Karaoglu MH (2011) TiO2 supported on sepiolite: preparation, structural and thermal characterization and catalytic behaviour in photocatalytic treatment of phenol and lignin from olive mill wastewater. Chem Eng J 166(3):859–867
Wolborska A (1989) Adsorption on activated carbon of p-nitrophenol from aqueous solution. Water Res 23:85–91
Yoon YH, Nelson JH (1984) Application of gas adsorption kinetics. Part I. A theoretical model for respirator catridge service time. Am Ind Hyg Assoc J 45:509–516
Acknowledgements
The authors gratefully acknowledge the financial support provided by the Japan Cooperation Center, Petroleum (JCCP) and the technical support of the JX Nippon Research Institute Co., Ltd. (JX-NRI). Special thanks are also due to Sami Abdulla, Riham Surkatti, and Ameera Fares for helping with setting up the experimental apparatus and data analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Guilherme L. Dotto
Rights and permissions
About this article
Cite this article
El-Naas, M.H., Alhaija, M.A. & Al-Zuhair, S. Evaluation of an activated carbon packed bed for the adsorption of phenols from petroleum refinery wastewater. Environ Sci Pollut Res 24, 7511–7520 (2017). https://doi.org/10.1007/s11356-017-8469-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8469-8