Abstract
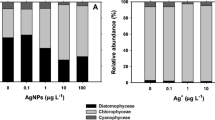
Although the industrial use of nanoparticles has increased over the past decade, the knowledge about their interaction with benthic phototrophic microorganisms in the environment is still limited. This study aims to characterize the toxic effect of ionic Ag+ and Ag nanoparticles (citrate-coated silver nanoparticles, AgNPs) in a wide concentration range (from 1 to 1000 μg L−1) and duration of exposure (2, 5 and 14 days) on three biofilm-forming benthic microorganisms: diatom Nitzschia palea, green algae Uronema confervicolum and cyanobacteria Leptolyngbya sp. Ag+ has a significant effect on the growth of all three species at low concentrations (1–10 μg L−1), whereas the inhibitory effect of AgNPs was only observed at 1000 μg L−1 and solely after 2 days of exposure. The inhibitory effect of both Ag+ and AgNPs decreased in the course of the experiments from 2 to 14 days, which can be explained by the progressive excretion of the exopolysaccharides and dissolved organic carbon by the microorganisms, thus allowing them to alleviate the toxic effects of aqueous silver. The lower impact of AgNPs on cells compared to Ag+ can be explained in terms of availability, internalization, reactive oxygen species production, dissolved silver concentration and agglomeration of AgNPs. The duration of exposure to Ag+ and AgNPs stress is a fundamental parameter controlling the bioaccumulation and detoxification in benthic phototrophic microorganisms.






Similar content being viewed by others
References
Albanese A, Tang PS, Chan WCW (2012) The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng 14:1–16. doi:10.1146/annurev-bioeng-071811-150124
Angel BM, Batley GE, Jarolimek CV, Rogers NJ (2013) The impact of size on the fate and toxicity of nanoparticulate silver in aquatic systems. Chemosphere 93:359–365. doi:10.1016/j.chemosphere.2013.04.096
Arulvasu C, Jennifer SM, Prabhu D, Chandhirasekar D (2014) Toxicity effect of silver nanoparticles in brine shrimp Artemia. Sci World J 2014:10. doi:10.1155/2014/256919
Auffan M, Rose J, Wiesner MR, Bottero J-Y (2009) Chemical stability of metallic nanoparticles: a parameter controlling their potential cellular toxicity in vitro. Environ Pollut 157:1127–1133
Baker TJ, Tyler CR, Galloway TS (2014) Impacts of metal and metal oxide nanoparticles on marine organisms. Environ Pollut 186:257–271. doi:10.1016/j.envpol.2013.11.014
Barnett BP, Arepally A, Karmarkar PV, Qian D, Gilson WD, Walczak P, Howland V, Lawler L, Lauzon C, Kraitchman DL, Bulte JWM (2007) Magnetic resonance-guided, real-time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islet cells. Nat Med 13:986–991. doi:10.1038/nm1581
Benn TM, Westerhoff P (2008) Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol 42:4133–4139
Blaser SA, Scheringer M, MacLeod M, Hungerbühler K (2008) Estimation of cumulative aquatic exposure and risk due to silver: contribution of nano-functionalized plastics and textiles. Sci Total Environ 390:396–409
Capek I (2004) Preparation of metal nanoparticles in water-in-oil (w/o) microemulsions. Adv Colloid Interf Sci 110:49–74
Choi O, Hu Z (2008) Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol 42:4583–4588
Cumberland SA, Lead JR (2009) Particle size distributions of silver nanoparticles at environmentally relevant conditions. J Chromatogr A 1216:9099–9105
Dobias J, Bernier-Latmani R (2013) Silver release from silver nanoparticles in natural waters. Environ Sci Technol 47:4140–4146. doi:10.1021/es304023p
Egorova EM (2011) Interaction of silver nanoparticles with biological objects: antimicrobial properties and toxicity for the other living organisms. J Phys Conf Ser 291:012050
El Badawy AM, Silva RG, Morris B, Scheckel KG, Suidan MT, Tolaymat TM (2010) Surface charge-dependent toxicity of silver nanoparticles. Environ Sci Technol 45:283–287. doi:10.1021/es1034188
Fabrega J, Fawcett SR, Renshaw JC, Lead JR (2009) Silver nanoparticle impact on bacterial growth: effect of pH, concentration, and organic matter. Environ Sci Technol 43:7285–7290. doi:10.1021/es803259g
Fabrega J, Luoma SN, Tyler CR, Galloway TS, Lead JR (2011a) Silver nanoparticles: behaviour and effects in the aquatic environment. Environ Int 37:517–531. doi:10.1016/j.envint.2010.10.012
Fabrega J, Zhang R, Renshaw JC, Liu W-T, Lead JR (2011b) Impact of silver nanoparticles on natural marine biofilm bacteria. Chemosphere 85:961–966. doi:10.1016/j.chemosphere.2011.06.066
Feng Q, Wu J, Chen G, Cui F, Kim T, Kim J (2000) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 52:662–668
Frattini A, Pellegri N, Nicastro D, Sanctis OD (2005) Effect of amine groups in the synthesis of Ag nanoparticles using aminosilanes. Mater Chem Phys 94:148–152. doi:10.1016/j.matchemphys.2005.04.023
Gélabert A, Pokrovsky OS, Schott J, Boudou A, Feurtet-Mazel A, Mielczarski J, Mielczarski E, Mesmer-Dudons N, Spalla O (2004) Study of diatoms/aqueous solution interface. I. Acid-base equilibria and spectroscopic observation of freshwater and marine species. Geochim Cosmochim Acta 68:4039–4058. doi:10.1016/j.gca.2004.01.011
Gil-Allué C, Schirmer K, Tlili A, Gessner MO, Behra R (2015) Silver nanoparticle effects on stream periphyton during short-term exposures. Environ Sci Technol 49:1165–1172
González A, Mombo S, Leflaive J, Lamy A, Pokrovsky O, Rols J-L (2015) Silver nanoparticles impact phototrophic biofilm communities to a considerably higher degree than ionic silver. Environ Sci Pollut Res 22:8412–8424. doi:10.1007/s11356-014-3978-1
González AG, Pokrovsky OS, Jiménez-Villacorta F, Shirokova LS, Santana-Casiano JM, González-Dávila M, Emnova EE (2014) Iron adsorption onto soil and aquatic bacteria: XAS structural study. Chem Geol 372:32–45. doi:10.1016/j.chemgeo.2014.02.013
González AG, Shirokova LS, Pokrovsky OS, Emnova EE, Martinez RE, Santana-Casiano JM, Gonzalez-Davila M, Pokrovski GS (2010) Adsorption of copper on Pseudomonas aureofaciens: protective role of surface exopolysaccharides. J Colloid Interface Sci 350:305–314
Gottschalk F, Sonderer T, Scholz RW, Nowack B (2009) Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ Sci Technol 43:9216–9222. doi:10.1021/es9015553
Gubbins EJ, Batty LC, Lead JR (2011) Phytotoxicity of silver nanoparticles to Lemna minor L. Environ Pollut 159:1551–1559
Gustafsson JP (2012) Visual MINTEQ, ver. 3.0. Compiled in visual basic. NET 2005. KYH, Dept of Land and Water Resources Engineering, Stockholm
He D, Dorantes-Aranda JJ, Waite TD (2012) Silver nanoparticle—algae interactions: oxidative dissolution, reactive oxygen species generation and synergistic toxic effects. Environ Sci Technol 46:8731–8738. doi:10.1021/es300588a
Huynh KA, Chen KL (2011) Aggregation kinetics of citrate and polyvinylpyrrolidone coated silver nanoparticles in monovalent and divalent electrolyte solutions. Environ Sci Technol 45:5564–5571
Ivask A, Kurvet I, Kasemets K, Blinova I, Aruoja V, Suppi S, Vija H, Käkinen A, Titma T, Heinlaan M, Visnapuu M, Koller D, Kisand V, Kahru A (2014) Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS One 9:e102108. doi:10.1371/journal.pone.0102108
Jiang J, Oberdörster G, Biswas P (2009) Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanopart Res 11:77–89
Kaegi R, Sinnet B, Zuleeg S, Hagendorfer H, Mueller E, Vonbank R, Boller M, Burkhardt M (2010) Release of silver nanoparticles from outdoor facades. Environ Pollut 158:2900–2905
Kalishwaralal K, BarathManiKanth S, Pandian SRK, Deepak V, Gurunathan S (2010) Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf B 79:340–344. doi:10.1016/j.colsurfb.2010.04.014
Kappler A, Newman DK (2004) Formation of Fe (III)-minerals by Fe (II)-oxidizing photoautotrophic bacteria. Geochim Cosmochim Acta 68:1217–1226
Kilham S, Kreeger D, Lynn S, Goulden C, Herrera L (1998) COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377:147–159. doi:10.1023/A:1003231628456
Kittler S, Greulich C, Diendorf J, Köller M, Epple M (2010) Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem Mater 22:4548–4554. doi:10.1021/cm100023p
Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin M, Lead JR (2008) Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem 27:1825–1851. doi:10.1897/08-090.1
Kroll A, Behra R, Kaegi R, Sigg L (2014) Extracellular polymeric substances (EPS) of freshwater biofilms stabilize and modify CeO2 and Ag nanoparticles. PLoS One 9:e110709
Kroll A, Matzke M, Rybicki M, Obert-Rauser P, Burkart C, Jurkschat K, Verweij R, Sgier L, Jungmann D, Backhaus T, Svendsen C (2016) Mixed messages from benthic microbial communities exposed to nanoparticulate and ionic silver: 3D structure picks up nano-specific effects, while EPS and traditional endpoints indicate a concentration-dependent impact of silver ions. Environ Sci Pollut Res 23:4218–4234. doi:10.1007/s11356-015-4887-7
Lee D-Y, Fortin C, Campbell PGC (2005) Contrasting effects of chloride on the toxicity of silver to two green algae, Pseudokirchneriella subcapitata and Chlamydomonas reinhardtii. Aquat Toxicol 75:127–135
Li X, Lenhart JJ (2012) Aggregation and dissolution of silver nanoparticles in natural surface water. Environ Sci Technol 46:5378–5386. doi:10.1021/es204531y
Liu W, Wu Y, Wang C, Li HC, Wang T, Liao CY, Cui L, Zhou QF, Yan B, Jiang GB (2010) Impact of silver nanoparticles on human cells: effect of particle size. Nanotoxicology 4:319–330. doi:10.3109/17435390.2010.483745
Lodeiro P, Achterberg EP, Pampín J, Affatati A, El-Shahawi MS (2016) Silver nanoparticles coated with natural polysaccharides as models to study AgNP aggregation kinetics using UV-visible spectrophotometry upon discharge in complex environments. Sci Total Environ 539:7–16. doi:10.1016/j.scitotenv.2015.08.115
López A, Rico M, Santana-Casiano JM, González AG, González-Dávila M (2015) Phenolic profile of Dunaliella tertiolecta growing under high levels of copper and iron. Environ Sci Pollut Res 22:14820–14828
Luoma SN, Ho YB, Bryan GW (1995) Fate, bioavailability and toxicity of silver in estuarine environments. Mar Pollut Bull 31:44–54
Marambio-Jones C, Hoek EV (2010) A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res 12:1531–1551. doi:10.1007/s11051-010-9900-y
Miao A-J, Luo Z, Chen C-S, Chin W-C, Santschi PH, Quigg A (2010) Intracellular uptake: a possible mechanism for silver engineered nanoparticle toxicity to a freshwater alga Ochromonas danica. PLoS One 5:e15196
Miao A-J, Schwehr KA, Xu C, Zhang S-J, Luo Z, Quigg A, Santschi PH (2009) The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environ Pollut 157:3034–3041. doi:10.1016/j.envpol.2009.05.047
Miller LA, Bruland KW (1995) Organic speciation of silver in marine waters. Environ Sci Technol 29:2616–2621. doi:10.1021/es00010a024
Morel FMM, Kustka AB, Shaked Y (2008) The role of unchelated Fe in the iron nutrition of phytoplankton. Limnol Oceanogr 53:400–404
Moreno-Garrido I, Pérez S, Blasco J (2015) Toxicity of silver and gold nanoparticles on marine microalgae. Mar Environ Res 111:60–73
Mueller NC, Nowack B (2008) Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol 42:4447–4453
Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao AJ, Quigg A, Santschi PH, Sigg L (2008a) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17:372–386
Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R, Odzak N, Sigg L, Behra R (2008b) Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ Sci Technol 42:8959–8964. doi:10.1021/es801785m
Nel A, Xia T, Mädler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311:622–627. doi:10.1126/science.1114397
Pal S, Tak YK, Song JM (2007) Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol 73:1712–1720
Park E-J, Yi J, Kim Y, Choi K, Park K (2010) Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol in Vitro 24:872–878
Park J-W, Oh J-H, Kim W-K, Lee S-K (2014) Toxicity of citrate-coated silver nanoparticles differs according to method of suspension preparation. Bull Environ Contam Toxicol 93:53–59. doi:10.1007/s00128-014-1296-4
Pavasupree S, Ngamsinlapasathian S, Nakajima M, Suzuki Y, Yoshikawa S (2006) Synthesis, characterization, photocatalytic activity and dye-sensitized solar cell performance of nanorods/nanoparticles TiO2 with mesoporous structure. J Photochem Photobiol A 184:163–169
Perelaer J, Hendriks CE, de Laat AW, Schubert US (2009) One-step inkjet printing of conductive silver tracks on polymer substrates. Nanotechnology 20:165303
Piccapietra F, Sigg L, Behra R (2011) Colloidal stability of carbonate-coated silver nanoparticles in synthetic and natural freshwater. Environ Sci Technol 46:818–825
Pillai S, Behra R, Nestler H, Suter MJF, Sigg L, Schirmer K (2014) Linking toxicity and adaptive responses across the transcriptome, proteome, and phenotype of Chlamydomonas reinhardtii exposed to silver. Proc Natl Acad Sci 111:3490–3495
Ribeiro FV (2014) Processos extremos na constituição da cidade. da crise à emergência nos espaços mundializados 2014. 18:2 doi:10.11606/issn.2179–0892.geousp.2014.81105
Rico M, López A, Santana-Casiano JM, González AG, González-Dávila M (2013) Variability of the phenolic profile in the diatom Phaeodactylum tricornutum growing under copper and iron stress. Limnol Oceanogr 58:144–152
Sikora FJ, Stevenson FJ (1988) Silver complexation by humic substances: conditional stability constants and nature of reactive sites. Geoderma 42:353–363. doi:10.1016/0016-7061(88)90011-0
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for gram-negative bacteria. J. Colloid Interface Sci 275:177–182. doi:10.1016/j.jcis.2004.02.012
Stanier R, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171
Tedesco S, Doyle H, Blasco J, Redmond G, Sheehan D (2010) Oxidative stress and toxicity of gold nanoparticles in Mytilus edulis. Aquat Toxicol 100:178–186. doi:10.1016/j.aquatox.2010.03.001
Tolaymat TM, El Badawy AM, Genaidy A, Scheckel KG, Luxton TP, Suidan M (2010) An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: a systematic review and critical appraisal of peer-reviewed scientific papers. Sci Total Environ 408:999–1006
Tungittiplakorn W, Lion LW, Cohen C, Kim J-Y (2004) Engineered polymeric nanoparticles for soil remediation. Environ Sci Technol 38:1605–1610. doi:10.1021/es0348997
van Schaik JWJ (2008) Binding of metals to macromolecular organic acids in natural waters vol 2008.
Wady AF, Machado AL, Foggi CC, Zamperini CA, Zucolotto V, Moffa EB, Vergani CE (2014) Effect of a silver nanoparticles solution on Staphylococcus aureus and Candida spp. J Nanomater 2014:128–128. doi:10.1155/2014/545279
Wei L, Thakkar M, Chen Y, Ntim SA, Mitra S, Zhang X (2010) Cytotoxicity effects of water dispersible oxidized multiwalled carbon nanotubes on marine alga, Dunaliella tertiolecta. Aquat Toxicol 100:194–201
Yin L, Cheng Y, Espinasse B, Colman BP, Auffan M, Wiesner M, Rose J, Liu J, Bernhardt ES (2011) More than the ions: the effects of silver nanoparticles on Lolium multiflorum. Environ Sci Technol 45:2360–2367. doi:10.1021/es103995x
Zhang W, Yao Y, Li K, Huang Y, Chen Y (2011) Influence of dissolved oxygen on aggregation kinetics of citrate-coated silver nanoparticles. Environ Pollut 159:3757–3762
Zhang W-X (2003) Nanoscale iron particles for environmental remediation: an overview. J Nanopart Res 5:323–332. doi:10.1023/A:1025520116015
Acknowledgments
This research was supported by the Midi-Pyrénées Regional Council (France) within the programme Gagilau (No. DAER-R93 90173). Partial support from BIO-GEO-CLIM grant No. 14.B25.31.0001 and ANR CITTOXIC-Nano are also acknowledged. Finally, we thank Katrin Meier for the English revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Thomas D. Bucheli
Electronic supplementary material
ESM 1
(DOC 826 kb)
Rights and permissions
About this article
Cite this article
González, A.G., Fernández-Rojo, L., Leflaive, J. et al. Response of three biofilm-forming benthic microorganisms to Ag nanoparticles and Ag+: the diatom Nitzschia palea, the green alga Uronema confervicolum and the cyanobacteria Leptolyngbya sp.. Environ Sci Pollut Res 23, 22136–22150 (2016). https://doi.org/10.1007/s11356-016-7259-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7259-z