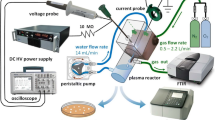
Abstract
The degradation of aqueous Rhodamine B (RhB) was examined using a dual-channel spark switch module designed to regulate the steepness of pulsed high voltage with microsecond rise time. Depending on the energy per pulse, a spark along the water surface (SPWS) or streamer along the water surface (STWS) was formed. STWS was found to have a better degradation effect and energy efficiency toward RhB than SPWS at the same power; however, addition of H2O2 amounts resulted in increased degradation, the effect being more pronounced using SPWS. The initial concentration of RhB also appeared to influence the rate constant of the degradation reaction. Furthermore, TiO2 films doped with Fe, Mn, and Ce were found to enhance the degradation performance of plasma. A possible reaction mechanism of plasma formation along the water surface was concluded by determination of the main inorganic products in the liquid and gas phases.
















Similar content being viewed by others
References
Alwash AH, Abdullah AZ, Ismail N (2012) Zeolite Y encapsulated with Fe-TiO2 for ultrasound-assisted degradation of amaranth dye in water. J Hazard Mater 233–234:184–193
Bagwasi S, Tian B, Zhang J, Nasir M (2013) Synthesis, characterization and application of bismuth and boron Co-doped TiO2: a visible light active photocatalyst. Chem Eng J 217:108–118
Barras A, Cordier S, Boukherroub R (2012) Fast photocatalytic degradation of rhodamine B over [Mo6Br8(N3)6]2− cluster units under sun light irradiation. Appl Catal, B 123–124:1–8
Burlica R, Shih KY, Locke BR (2010) Formation of H2 and H2O2 in a water-spray gliding arc nonthermal plasma reactor. Ind Eng Chem Res 49(14):6342–6349
Cataldo F, Compagnini G, D’Urso L, Mita V, Strazzulla G, Ursini O, Angelini G (2008) Adsorption of dinitrogen tetroxide (N2O4) on multi‐walled carbon nanotubes (MWCNTs). Fullerenes, Nanotubes, Carbon Nonstructures 16(2):154–164
Chiou C, Juang R (2007) Photocatalytic degradation of phenol in aqueous solutions by Pr-doped TiO2 nanoparticles. J Hazard Mater 149(1):1–7
Colmenares JC, Magdziarz A, Kurzydlowski K, Grzonka J, Chernyayeva O, Lisovytskiy D (2013) Low-temperature ultrasound-promoted synthesis of Cr-TiO2-supported photocatalysts for valorization of glucose and phenol degradation from liquid phase. Appl Catal B-Environ 134:136–144
Dojčinović BP, Roglić GM, Obradović BM, Kuraica MM, Kostić MM, Nešić J, Manojlović DD (2011) Decolorization of reactive textile dyes using water falling film dielectric barrier discharge. J Hazard Mater 192(2):763–771
Ghezzar MR, Abdelmalek F, Belhadj M, Benderdouche N, Addou A (2007) Gliding arc plasma assisted photocatalytic degradation of anthraquinonic acid green 25 in solution with TiO2. Appl Catal, B 72(3–4):304–313
Guo J, Ma B, Yin A, Fan K, Dai W (2011) Photodegradation of rhodamine B and 4-chlorophenol using plasmonic photocatalyst of Ag–AgI/Fe3O4@SiO2 magnetic nanoparticle under visible light irradiation. Appl Catal, B 101(3–4):580–586
Gupta, S. B (2007) Investigation of a physical disinfection process based on pulsed underwater corona discharges. In Forschungszentrum Karlsruhe
Hu H, Xiao W, Yuan J, Shi J, He D, Shangguan W (2008) High photocatalytic activity and stability for decomposition of gaseous acetaldehyde on TiO2/Al2O3 composite films coated on foam nickel substrates by sol-gel processes. J Sol-Gel Sci Technol 45(1):1–8
Jan ZY, Ren WZ, Wang X (2011) Study on the decolorizing of Rhodamine B by the liquid discharge on Ti anode. Computer Science and Service System, International Conference on 2011, pp 3607–3610
Kaji T, Kawashima T, Yamamoto C, Sakamoto M (1992) Rhodamine B inhibits collagen synthesis by human lip fibroblasts in culture. Toxicol Lett 61(1):81–87
Kolb JF, Mohamed A, Price RO, Swanson RJ, Bowman A, Chiavarini RL, Stacey M, Schoenbach KH (2008) Cold atmospheric pressure air plasma jet for medical applications. Appl Phys Lett 92(24):241501
Kušić H, Koprivanac N, Locke BR (2005) Decomposition of phenol by hybrid gas/liquid electrical discharge reactors with zeolite catalysts. J Hazard Mater 125(1–3):190–200
Le TT, Akhtar MS, Park DM, Lee JC, Yang O (2012) Water splitting on Rhodamine-B dye sensitized Co-doped TiO2 catalyst under visible light. Appl Catal, B 111–112:397–401
Lee H, Park SH, Park YK, Kim BH, Kim SJ, Jung SC (2013) Rapid destruction of the Rhodamine B using TiO2 photocatalyst in the liquid phase plasma. Chem Central J 7(1):156
Li W, Zhao S, Qi B, Du Y, Wang X, Huo M (2009) Fast catalytic degradation of organic dye with air and MoO3:Ce nanofibers under room condition. Appl Catal, B 92(3–4):333–340
Lu Q, Gao W, Du J, Zhou L, Lian Y (2012) Discovery of environmental Rhodamine B contamination in paprika during the vegetation process. J Agric Food Chem 60(19):4773–4778
Manoj Kumar Reddy P, Rama Raju B, Karuppiah J, Linga Reddy E, Subrahmanyam C (2013) Degradation and mineralization of methylene blue by dielectric barrier discharge non-thermal plasma reactor. Chem Eng J 217:41–47
Narengerile H, Yuan M, Watanabe T (2011) Decomposition mechanism of phenol in water plasmas by DC discharge at atmospheric pressure. Chem Eng J 168(3):985–993
Ni GH, Zhao GX, Jiang YM, Li JX, Meng YD, Wang XK (2013) Steam plasma jet treatment of phenol in aqueous solution at atmospheric pressure. Plasma Processes Polym 10(4):353–363
Noeske M, Degenhardt J, Strudthoff S, Lommatzsch U (2004) Plasma jet treatment of five polymers at atmospheric pressure: surface modifications and the relevance for adhesion. Int J Adhes Adhes 24(2):171–177
Othman I, Mohamed RM, Ibrahem FM (2007) Study of photocatalytic oxidation of indigo carmine dye on Mn-supported TiO2. J Photochem Photobiol, A 189(1):80–85
Parshetti GK, Doong R (2011) Synergistic effect of nickel ions on the coupled dechlorination of trichloroethylene and 2,4-dichlorophenol by Fe/TiO2 nanocomposites in the presence of UV light under anoxic conditions. Water Res 45(14):4198–4210
Sato M, Tokutake T, Ohshima T, Sugiarto AT (2008) Aqueous phenol decomposition by pulsed discharges on the water surface. Ind Appl, IEEE Trans 44(5):1397–1402
Shaban YA, El Sayed MA, El Maradny AA, Al Farawati RK, Al Zobidi MI (2013) Photocatalytic degradation of phenol in natural seawater using visible light active carbon modified (CM)-n-TiO2 nanoparticles under UV light and natural sunlight illuminations. Chemosphere 91(3):307–313
Shiota H, Itabashi H, Satoh K, Itoh H (2013) Phenol decomposition by pulsed-discharge plasma above a water surface in oxygen and argon atmosphere. Electr Eng Japan 184(1):1–9
Smith DM, Welch WF, Graham SM, Chughtai AR, Wicke BG, Grady KA (1988) Reaction of nitrogen oxides with black carbon: an FT-IR study. Appl Spectrosc 42(4):674–680
Souzanchi S, Vahabzadeh F, Fazel S, Hosseini SN (2013) Performance of an annular sieve-plate column photoreactor using immobilized TiO2 on stainless steel support for phenol degradation. Chem Eng J 223:268–276
Sugiarto AT, Ohshima T, Sato M (2002) Advanced oxidation processes using pulsed streamer corona discharge in water. Thin Solid Films 20(1):174–178
Sugiarto AT, Ito S, Ohshima T, Sato M, Skalny JD (2003) Oxidative decoloration of dyes by pulsed discharge plasma in water. J Electrost 58(1–2):135–145
Tada H, Tanaka M (1997) Dependence of TiO2 photocatalytic activity upon its film thickness. Langmuir 13(2):360–364
Tryba B (2008) Immobilization of TiO2 and Fe–C–TiO2 photocatalysts on the cotton material for application in a flow photocatalytic reactor for decomposition of phenol in water. J Hazard Mater 151(2–3):623–627
Wang L, Jiang X (2009) Unusual catalytic effects of iron salts on phenol degradation by glow discharge plasma in aqueous solution. J Hazard Mater 161(2–3):926–932
Wang H, Li J, Quan X, Wu Y, Li G, Wang F (2007) Formation of hydrogen peroxide and degradation of phenol in synergistic system of pulsed corona discharge combined with TiO2 photocatalysis. J Hazard Mater 141(1):336–343
Yu J, Zhao X, Zhao Q (2000) Effect of film thickness on the grain size and photocatalytic activity of the sol-gel derived nanometer TiO2 thin films. J Mater Sci Lett 19(12):1015–1017
Zhang R, Zhang C, Cheng X, Wang L, Wu Y, Guan Z (2007) Kinetics of decolorization of azo dye by bipolar pulsed barrier discharge in a three-phase discharge plasma reactor. J Hazard Mater 142(1–2):105–110
Zhang Y, Xin Q, Cong Y, Wang Q, Jiang B (2013) Application of TiO2 nanotubes with pulsed plasma for phenol degradation. Chem Eng J 215–216:261–268
Zheng C, Xu Y, Huang H, Zhang Z, Liu Z, Yan K (2013) Water disinfection by pulsed atmospheric air plasma along water surface. Am Inst Chem Eng 59(5):1458–1467
Acknowledgments
This work is sponsored by the National Science Foundation of China (No. 11005091) and Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Rights and permissions
About this article
Cite this article
Chen, Y., Li, Y., Zhu, A. et al. Degradation of aqueous Rhodamine B by plasma generated along the water surface and its enhancement using nanocrystalline Fe-, Mn-, and Ce-doped TiO2 films. Environ Sci Pollut Res 21, 9948–9958 (2014). https://doi.org/10.1007/s11356-014-2982-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2982-9