Abstract
Purpose
The inflammatory markers chitinase-3-like protein 1 (CHI3L1) and chitotriosidase (CHIT1) have both been associated with cardiovascular complications. The aim of this preliminary observational study was to assess the roles and interaction of obstructive sleep apnea (OSA) severity and body mass index (BMI) with plasma CHI3L1 levels and CHIT1 activity in patients with moderate to severe OSA. The second aim was to assess the roles and interaction of positive airway pressure (PAP) treatment and BMI on the expression of the same proteins.
Methods
The study included 97 OSA patients with an apnea–hypopnea index (AHI) ≥ 15 and full usage of PAP treatment after 4 months. Plasma CHI3L1 levels and CHIT1 activity were measured before and after treatment.
Results
Multiple linear regression analysis demonstrated an independent association of BMI on CHI3L1 levels (p < 0.05) but not on CHIT1 activity. The OSA severity markers (AHI and oxygen desaturation index) did not independently or in interaction with BMI levels associate with CHI3L1 levels or with CHIT1 activity (p > 0.05). A two-way repeated measures ANOVA revealed a significant interaction between PAP treatment effect (before vs. after) and BMI groups (< 35 kg/m2 vs. ≥ 35 kg/m2) on CHI3L1 levels (p = 0.03) but not on CHIT1 activity (p = 0.98).
Conclusions
Obesity independently associated with CHI3L1 levels. Association between OSA severity and CHI3L1 levels or CHIT1 activity (independent of or dependent on obesity level) could not be confirmed. However, decrease was observed in CHI3L1 levels after PAP treatment in severely obese OSA patients but not in those less obese.
Similar content being viewed by others
Introduction
Obstructive sleep apnea (OSA) is a common disorder characterized by periodic reduction (hypopnea) or cessation (apnea) of airflow due to narrowing of the upper airway during sleep. OSA can effectively be treated with positive airway pressure (PAP) treatment [1]. Untreated OSA patients are at increased risk of developing cardiovascular complications such as hypertension, stroke, and cardiovascular disease [2].
Obesity is an important risk factor for both OSA and cardiovascular events. Obesity and OSA also activate similiar biological pathways of sympathetic activity, oxidative stress, and low-grade systemic inflammation [3]. It has therefore been a challenge separating the independent roles of OSA on cardiovascular disorders from those of obesity. It is likely that the role of OSA in cardiovascular disease is to some degree dependent on obesity. In our previous publications [4,5,6], we have found a moderating effect of obesity on how OSA affects both inflammatory-related protein levels, with the largest effect of OSA in severely obese patients with body mass index (BMI) ≥ 35 kg/m2. The concept that interaction between OSA and obesity increases inflammatory response could shape the design of future treatment clinical trials [3]. The discovery of biomarkers measuring this interaction effect is therefore of importance as it has the potential of providing important information about treatment response.
Chitinase-3-like protein 1 (CHI3L1, also known as YKL-40) and chitotriosidase (CHIT1) are proteins expressed in response to inflammation and have both been associated with cardiovascular disorders [7]. CHI3L1 is a 40-kD glycoprotein of the glycosyl hydrolase 18 family, secreted by activated macrophages, chondrocytes, neutrophils, and synovial cells [8]. The protein is one of four inactive chitinases that bind to chitin, but has no chitinase activity [9]. Elevated CHI3L1 levels have been associated with aging [10], degree of obesity [11], diabetes, and cardiovascular complications including atherosclerosis, acute myocardial infarction, and coronary artery disease [12]. A few studies [13,14,15,16] have also linked increased CHI3L1 levels to the presence and severity of OSA, although more research is still needed. CHI3L1 is secreted locally at the site of inflammation and not in response to systemic inflammation like some inflammatory markers (e.g., C-reactive protein [CRP]), indicating it could be of additional clinical use [17]. As an example, one study investigated the influence of statin treatment on CHI3L1 and high-sensitivity CRP levels in patients with stable ischemic heart disease (IHD) [18]. The statin-induced reduction of the two inflammatory markers was much more prominent for CHI3L1 than CRP, indicating difference between the two biomarkers in relation to their ability to monitor the inflammatory response in patients with IHD.
CHIT1 is a 50-kD mammalian chitinase of the same family. Unlike CHI3L1, CHIT1 is an active enzyme, mainly secreted by active macrophages and epithelial cells [19]. Previous studies have demonstrated that high CHIT1 activity is associated with Gaucher’s disease, atherosclerosis, and type 2 diabetes [20]. Very little is known about the relationship between CHIT1 activity and sleep. One previous study [21] linked CHIT1 activity to insufficient sleep in mice and rats. To our best knowledge, only one study has investigated the relationship between CHIT1 and OSA. Tamanaha et al. [22] tested whether plasma CHIT1 activity was related to the presence and severity of OSA, with results not indicating a significant association. Similarly, little is known about the relationship between CHIT1 and obesity. Alanbay et al. [23] found no significant correlation between CHIT1 activity and BMI.
No studies are available assessing the effect of PAP treatment on change in CHI3L1 levels or CHIT1 activity. The aim of this preliminary study was to evaluate the roles and interaction of OSA severity (AHI, oxygen desaturation index [ODI]), and obesity (BMI) on CHI3L1 levels and CHIT1 activity in a clinical cohort of patients with moderate to severe OSA (AHI ≥ 15). The second aim was to assess the roles and interaction of positive airway pressure (PAP) treatment and BMI on the expression of the same proteins.
Methods
Participants
A subset of 97 participants were selected from a larger 284 patients study [24]. All the participants had been diagnosed with OSA in Iceland and referred for PAP treatment to the Landspitali—The National University Hospital in Iceland from February 2010 to December 2013. The initial diagnosis of OSA was defined by AHI ≥ 15 events/h and oxygen desaturation index (ODI) ≥ 10 events/h. When sleep studies were rescored, there were however some subjects (n = 9) who had AHI between 10 and 15 events/h but they were not excluded from the study. More than 90% of approached subjects agreed to participate. Selection criteria for the subset used in this study were as follows: (1) participation in a sleep study before starting PAP treatment with a recorded apnea–hypopnea index (AHI) and oxygen desaturation index (ODI). All participants had moderate to severe OSA (defined as AHI ≥ 15) as that was a requirement for PAP treatment; (2) having blood samples taken while untreated and at follow-up; (3) used PAP treatment for ≥ 20 days and ≥ 4 h per day on average for the previous 4 weeks; and (5) completion of a 4-month follow-up as of May 13, 2014. This study was approved by the National Bioethics Committee and the Data Protection Authority of Iceland (10-048). Written consent was obtained from all subjects.
Questionnaires and measurements
A core questionnaire on sleep, health, and medication use was answered by subjects at baseline and at a 4-month follow-up (e.g., if they had been diagnosed with hypertension, diabetes or cardiovascular disease, or other diseases by a physician). Height and weight were measured for all participants with the same instruments (a ruler and a scale), after having removed shoes as well as objects from clothes. Blood was drawn in the morning from the antecubital vein after overnight fasting, both at baseline from untreated participants and at follow-up. Prior to referral for PAP treatment, all subjects had a type 3 sleep study with a T3 device (Nox Medical, Reykjavik, Iceland), an Embletta type 3 portable monitor, or an Embla 12-channel system (Natus Medical Inc., San Carlos, CA, USA). Type 3 portable monitors were used for home sleep apnea testing (HSAT) as this is the clinical practice in Iceland as it is in many other countries, e.g., in Europe [25], and accepted by the AASM in the USA [26]. The study therefore lacks electroencephalographic recording and assessment of arousals. However, the validity of the Nox T3 portable monitoring system for assessing sleep-disordered breathing has been shown when compared with polysomnography with similar AHI and ODI levels [27, 28]. Also, the respiratory scoring rules used (requiring 4% oxygen desaturation for hypopnea events) do not require arousals to assess hypopneas. The same signals were recorded on all studies. Nasal airflow was recorded through a cannula. Chest and abdominal movements were measured using respiratory inductance plethysmography belts. Pulse and oxygen desaturation were measured by a finger probe oximeter based on a four-beat exponential average (Nonin Medical Inc., Plymouth, MN, USA). Body position and activity were measured using sensors situated on the chest. The sleep studies were scored by trained sleep technologists. Studies had to have ≥ 4 h of scorable oxygen saturation and more than two out of three respiratory traces: cannula flow, thorax and respiratory inductive plethysmography belts. Total analysis time was assessed based on questionnaires and the sleep technologist’s review of the study. Sleep studies were scored in accordance with the American Academy of Sleep Medicine (AASM) 2007 manual [29], using the recommended hypopnea classification requiring a ≥ 30% drop in respiratory flow for ≥ 10 s with ≥ 4% oxygen desaturation.
PAP use
PAP adherence at follow-up was measured objectively by downloading the mask-on time stored by the PAP unit in the previous 4 weeks (S8 machines, ResMed, San Diego, CA, USA). Participants who used PAP for ≥ 20 days and ≥ 4 h per day on average for the previous 4 weeks were considered full users.
Biomarker assessment
Blood was collected in BD vacutainers containing EDTA (BD, Franklin Lakes, NJ, USA), gently inverted 8–10 times, and placed on ice. Within 1 h of collection, the sample was spun for 15 min at 1790g in a refrigerated centrifuge. The samples were kept on ice during aliquoting. After separation, the plasma samples were stored at − 80 °C.
The CHI3L1 levels in human plasma samples were measured using a commercially available Sandwich Elisa Duoset from R&D systems (cat. DY2599, Minneapolis, MN). The assays were run in 96-well plates (Nunc 442404, MaxiSorp) using 100 μl of plasma (1:800 dilution) as per manufacturer’s instructions. For standards, human recombinant CHI3L1 protein was used at concentrations ranging from 31.25 to 2000 pg/ml. All samples were measured in duplicates.
Chitotriosidase enzyme assay was based on the method described by Hollak et al. [30] with minor modifications. Briefly, chitotriosidase activity was determined by incubating 5 μl of plasma with 100 μl of 22 mol/l fluorogenic substrate 4-methylumbelliferyl β-d-N,N′,N′′-triacetylchitotrioside (Sigma M5639) in 0.1 M/0.2 M citrate–phosphate buffer (pH 5.2) for 15 min at 37 °C. The reaction was stopped with 200 μl of 1 M glycine–NaOH buffer (pH 10.6) by mixing at room temperature. The substrate hydrolysis by chitotriosidase produces the fluorescent molecule 4-methylumbelliferone, which was quantified with a fluorometer (Spectramax M3 instrument), excitation at 360 nm and emission at 450 nm, and compared with a standard 4-methylumbelliferone (Sigma M1508) calibration curve. Plasma chitotriosidase activity was measured in triplicates. Inter-assay coefficient of variation (CV) for both CHI3L1 and CHIT1 was within 20% and intra-assay CV was within 10%.
Statistical analysis
Descriptive group comparisons were performed using one-way analysis of variance and chi-square tests for continuous and categorical variables, respectively. CHI3L1 and CHIT1 were natural log-transformed for all analyses (based on assessment of residual error distribution, thereby permitting parametric analysis). The strengths of linear associations among biomarker values (CHI3L1 levels, CHIT1 activity), BMI, and OSA severity were assessed using Pearson’s correlation and multiple linear regression. Paired t test was used to compare the means of biomarker values before and after PAP treatment. Independent t test was used to compare the means of biomarker values between men and women. A two-way repeated measures ANOVA with interaction effects was performed to evaluate interaction effects between PAP treatment and BMI groups on biomarker values. All statistical analyses were performed using SPSS v.23.0 (IBM Corp., Armonk, NY, USA).
To avoid extrapolation beyond the scope of the data, sensitivity analyses were performed excluding those participants who had OSA severity not found in the other BMI groups [3]. All of the analyses led to the same conclusions as the original ones, and therefore all participants were used for the final analyses of the study.
Results
Baseline clinical and biochemical characteristics
Clinical and biochemical characteristics of OSA patients at baseline, both overall and within different BMI categories, are presented in Table 1. Severely obese subjects (BMI ≥ 35 kg/m2) had more severe OSA (higher AHI and ODI) as well as higher levels of CHI3L1, compared to patients with lower BMI (< 30 kg/m2 and 30–35 kg/m2). Furthermore, OSA patients within the highest BMI group also had a higher prevalence of type 2 diabetes compared to the other groups.
The range in values for AHI was between 15 and 116 events/h; for ODI, between 4 and 113 event/h; and for BMI, between 24.8 and 53.3 kg/m2.
Association between OSA severity and obesity with chitinase levels and activity
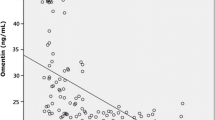
The correlation between chitinases and OSA severity markers (AHI, ODI) as well as obesity levels (BMI) was assessed. BMI was positively correlated with CHI3L1 levels (r = 0.21, p = 0.04) but not with CHIT1 activity (r = 0.06, p = 0.53). For OSA severity markers, a tendency for a weak positive correlation was observed with CHI3L1 levels, although not significant, and no relationship was found with CHIT1 activity (Fig. 1).
The relationships between the OSA severity markers and the chitinases were also assessed within the three different BMI categories. An indication of a specific pattern appeared for CHI3L1 levels. Although all insignificant (p > 0.05), both OSA severity markers correlated negatively with CHI3L1 levels within the lower BMI groups (< 30 kg/m2 and 30–35 kg/m2) but positively within the highest BMI group (BMI ≥ 35 kg/m2, Fig. S1a and Fig. S2a in supplement). No effect between OSA severity and CHIT1 activity was found when assessed within the three the same BMI categories (Fig. S1b and Fig. S2b in supplement).
To further evaluate how the level of BMI affects the relationship between OSA severity and chitinases, multiple linear regression analysis with interaction was performed (Table 2). The analysis demonstrated independent association of BMI and age with CHI3L1 levels. Significance (p < 0.05) was reached, independent of AHI (model 1) or ODI (model 2) being included in the analysis. Neither AHI nor ODI associated independently with CHI3L1 levels and the interaction between the markers and BMI was also non significant (p > 0.05). No independent association was found with CHIT1 activity.
The effect of PAP treatment on chitinase levels and activity
Overall, no significant differences in CHI3L1 levels (3.87 ± 0.05 vs. 3.79 ± 0.05, p = 0.08) and CHIT1 activity (3.17 ± 0.09 vs. 3.20 ± 0.09, p = 0.08) were found among OSA patients before and after PAP treatment. There was a trend towards an interaction between treatment and BMI, although the interaction effect did not reach significance (p = 0.08, Fig. S3 in supplement). For an increased power, we also ran the analysis by combining the two lower BMI groups. Figure 2 presents differences in CHI3L1 levels and CHIT1 activity before and after PAP treatment when stratified by two BMI groups (< 35 kg/m2 and ≥ 35 kg/m2). A two-way repeated measures ANOVA revealed a significant interaction (p = 0.028) between the two factors for CHI3L1 levels showing a significant decrease in CHI3L1 levels for OSA patients with PAP treatment with BMI ≥ 35 only, not those with BMI < 35 (Fig. 2a). No significant interaction between PAP treatment and BMI groups (p = 0.976) was found for CHIT1 activity (Fig. 2b).
Four additional repeated measures ANOVA analyses were also performed, with each analysis excluding a different group of patients from the study, to assess the potential confounding effects of other diseases. Each analysis excluded patients with hypertension, cardiovascular disease, stroke, or type 2 diabetes. Interaction effects between PAP treatment and the two BMI groups reached significance for CHI3L1 levels (p < 0.05) but not for CHIT1 activity, independent of which patient group was excluded. This is in accordance with the primary analysis. No significant differences were found between men and women in CHI3L1 levels at baseline (3.86 ± 0.06 vs. 3.87 ± 0.10, p = 0.99) or after treatment (3.78 ± 0.06 vs. 3.82 ± 0.10, p = 0.72). Despite this lack of difference, sensitivity analysis was performed including men only (n = 72). The interaction effect for CHI3L1 levels showed a similar trend as for the main analysis but did not reach significance (p = 0.20, Fig. S4 in supplement).
Discussion
The results of this study indicate that effects of PAP treatment on CHI3L1 levels depend on the level of obesity (measured by BMI) in a population of patients with moderate to severe OSA. Obesity did also positively associate with the concentration of the same protein. The effect of OSA severity on CHI3L1 levels, with and without the degree of obesity, was not significant indicating that the AHI and ODI as markers of OSA severity may not be appropriate to assess the impact of OSA on CHI3L1 levels in the same patient populations. Furthermore, the effect of PAP treatment, degree of obesity, and OSA severity did not associate with CHIT1 activity.
Few recent studies have demonstrated an independent association of both the presence and severity of OSA on serum CHI3L1 levels [13,14,15,16]. Our results are not in accordance with these previous studies as an independent relationship between OSA severity and CHI3L1 levels could not be confirmed. A possible explanation is that our sample did only include patients with moderate to severe OSA, not those with mild OSA and healthy controls. Our results, on the other hand, confirmed an independent, positive association of obesity and age with CHI3L1 levels, which has also been reported in earlier studies [10, 11, 31].
Obesity is an important risk factor in the pathogenesis of OSA [32,33,34]. As obesity and OSA tend to coexist, they can also independently affect similar biological pathways such as oxidative stress and inflammation [35,36,37,38]. Previous studies have indicated an accumulation of activated macrophages and other immune active cells in visceral adipose tissue from obese subjects as possible sources of inflammatory cytokines, determining a link between obesity and low-grade inflammation [39, 40]. It could therefore be hypothesized that the effect of OSA is amplified by the increased number of inflammatory cells in fat in the most obese patients [41]. Arnardottir et al. [4] demonstrated, in a large study based on the Icelandic Sleep Apnea Cohort, that the independent association of OSA with levels of pro-inflammatory cytokines interleukin-6 (IL-6), and CRP depended on the degree of obesity. These results were furthermore confirmed in a long-term follow-up [5] where the increase of interleukin-6 was prevented by PAP treatment, but only among severely obese patients (BMI ≥ 35 kg/m2). CHI3L1 has been identified as a major protein expressed in macrophages among stromal vascular fraction cells in human visceral adipose tissue [42]. When assesing the role of PAP treatment and obesity on CHI3L1 levels, our results came to similar conclusions with these studies. Change in levels of the protein after PAP treatment did depend on degree of obesity. Reduction in CHI3L1 levels was found among severely obese patients (BMI ≥ 35 kg/m2) after treatment but not in those less obese (BMI < 35 kg/m2).
An interaction effect did not appear between PAP treatment and obesity regarding CHIT1, as there were no significant changes in activity after treatment when analyzed by different BMI categories. Very few studies have examined the relationship between CHIT1 enzyme activity and sleep. A previous study by Lungato et al. [21] showed association between CHIT1 plasma levels and paradoxical sleep deprivation in adult male mice and rats. They found a significant increase in CHIT1 plasma levels when the animals were subjected to paradoxical sleep deprivation for 72 h compared to controls. Because sleep has an important role for the maintenance and efficiency of the immune system [43, 44], their data suggested a relationship between sleep and macrophage response, where CHIT1 activity could possibly serve as a marker for insufficient sleep. To our best knowledge, only one study has investigated the relationship between OSA and CHIT1. Tamanaha et al. [22] tested whether plasma CHIT1 activity was related to the presence and severity of OSA in a Brazilian cohort, where the results did not indicate a significant association. Our results are in accordance with those results: no association was measured between OSA severity and CHIT1 activity. The relationship did not appear to depend on obesity either, with no specific interaction effect appearing. To our best knowledge, it has not been tested if the enzyme is expressed by macrophages and endothelial cells in the visceral adipose tissue.
There are a few major limitations of the present study. First, this is an observational study, not a randomized controlled trial, with all participants receiving full PAP treatment. Therefore, our findings are preliminary and have to be interpreted with caution. Second, this was a study with a relatively small number of OSA patients and present findings need to be validated in a larger study. The sample did not include non-OSA controls or mild OSA patients and the limited variability in the lower end of OSA severity and BMI could underestimate associations between the studied variables. The sample was also relatively homogeneous with Caucasian and mostly male participants. The results might therefore not generalize to other more ethnically diversed populations or across gender. No difference in serum CHI3L1 levels between males and females have though been found in previous studies [45]. Third, we did not have other measurements of obesity like visceral fat and waist circumference, change in BMI during the 4-month treatment period, or concentrations of other pro-inflammatory mediators, which may provide valuable information on a possible role of CHI3L1 and CHIT1 in the progression of OSA. However, our previous paper assessing the role of different fat measures in inflammatory biomarkers showed that total fat measures such as BMI were more highly associated with inflammatory levels than visceral fat levels per se [4]. Fourth, with multiple sensitivity analyses (excluding each disease), there is an increased risk of false positive results (type 1 error). By replicating the study in a larger sample, more information could be fitted into the statistical model, decreasing the risk of this type of error. Also, with a larger sample, estimation of coefficients will be more precise. Finally, residual confounding cannot be fully excluded as confounding factors were assessed with crude precision (e.g., having a condition vs. not having a condition).
In conclusion, this is the first paper to assess the effect of PAP treatment on CHI3L1 levels and CHIT1 activity in OSA patients. The results show a decrease in CHI3L1 levels after 4 months of full PAP treatment in severely obese OSA patients (BMI ≥ 35 kg/m2), but not in those less obese (BMI < 35 kg/m2). The same effect was not observed with CHIT1 activity. Obesity and age were independently associated with CHI3L1 levels but not with CHIT1 acitvity. Association between OSA severity and CHI3L1 levels/CHIT1 activity (independent of or dependent on obesity) could not be confirmed.
References
Marin JM, Carrizo SJ, Vicente E, Agusti AG (2005) Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study, Lancet (London, England). 365(9464):1046–1053. https://doi.org/10.1016/s0140-6736(05)71141-7
Young T, Peppard PE, Gottlieb DJ (2002) Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 165(9):1217–1239
Pack AI, Gislason T (2009) Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Prog Cardiovasc Dis 51(5):434–451. https://doi.org/10.1016/j.pcad.2009.01.002
Arnardottir ES, Maislin G, Schwab RJ, Staley B, Benediktsdottir B, Olafsson I, Juliusson S, Romer M, Gislason T, Pack AI (2012) The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: the Icelandic Sleep Apnea Cohort. Sleep 35(7):921–932. https://doi.org/10.5665/sleep.1952
Pak VM, Keenan BT, Jackson N, Grandner MA, Maislin G, Teff K, Schwab RJ, Arnardottir ES, Juliusson S, Benediktsdottir B, Gislason T, Pack AI (2015) Adhesion molecule increases in sleep apnea: beneficial effect of positive airway pressure and moderation by obesity. Int J Obes (2005) 39(3):472–479. https://doi.org/10.1038/ijo.2014.123
Arnardottir ES, Lim DC, Keenan BT, Maislin G, Benediktsdottir B, Juliusson S, Pack AI, Gislason T (2015) Effects of obesity on the association between long-term sleep apnea treatment and changes in interleukin-6 levels: the Icelandic Sleep Apnea Cohort. J Sleep Res 24(2):148–159. https://doi.org/10.1111/jsr.12252
Jiang H, Schiffer E, Song Z, Wang J, Zurbig P, Thedieck K, Moes S, Bantel H, Saal N, Jantos J, Brecht M, Jeno P, Hall MN, Hager K, Manns MP, Hecker H, Ganser A, Dohner K, Bartke A, Meissner C, Mischak H, Ju Z, Rudolph KL (2008) Proteins induced by telomere dysfunction and DNA damage represent biomarkers of human aging and disease. Proc Natl Acad Sci U S A 105(32):11299–11304. https://doi.org/10.1073/pnas.0801457105
Bonneh-Barkay D, Bissel SJ, Wang G, Fish KN, Nicholl GC, Darko SW, Medina-Flores R, Murphey-Corb M, Rajakumar PA, Nyaundi J, Mellors JW, Bowser R, Wiley CA (2008) YKL-40, a marker of simian immunodeficiency virus encephalitis, modulates the biological activity of basic fibroblast growth factor. Am J Pathol 173(1):130–143. https://doi.org/10.2353/ajpath.2008.080045
Hakala BE, White C, Recklies AD (1993) Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem 268(34):25803–25810
Bojesen SE, Johansen JS, Nordestgaard BG (2011) Plasma YKL-40 levels in healthy subjects from the general population. Clin Chim Acta 412(9–10):709–712. https://doi.org/10.1016/j.cca.2011.01.022
Hempen M, Kopp HP, Elhenicky M, Hobaus C, Brix JM, Koppensteiner R, Schernthaner G, Schernthaner GH (2009) YKL-40 is elevated in morbidly obese patients and declines after weight loss. Obes Surg 19(11):1557–1563. https://doi.org/10.1007/s11695-009-9917-4
Rathcke CN, Vestergaard H (2009) YKL-40--an emerging biomarker in cardiovascular disease and diabetes. Cardiovasc Diabetol 8:61. https://doi.org/10.1186/1475-2840-8-61
Wang X, Xing GH (2014) Serum YKL-40 concentrations are elevated and correlated with disease severity in patients with obstructive sleep apnea syndrome. Scand J Clin Lab Invest 74(1):74–78. https://doi.org/10.3109/00365513.2013.859726
Jafari B, Elias JA, Mohsenin V (2014) Increased plasma YKL-40/chitinase-3-like-protein-1 is associated with endothelial dysfunction in obstructive sleep apnea. PLoS One 9(5):e98629. https://doi.org/10.1371/journal.pone.0098629
Li W, Yu Z, Jiang C (2014) Association of serum YKL-40 with the presence and severity of obstructive sleep apnea syndrome. Lab Med 45(3):220–225. https://doi.org/10.1309/lms98akcxrsl2xor
Bakirci EM, Unver E, Degirmenci H, Kivanc T, Gunay M, Hamur H, Buyuklu M, Ceyhun G, Topal E, Coban TA (2015) Serum YKL-40/chitinase 3-like protein 1 level is an independent predictor of atherosclerosis development in patients with obstructive sleep apnea syndrome. Turk Kardiyoloji Dernegi arsivi : Turk Kardiyoloji Derneginin yayin organidir 43(4):333–339. https://doi.org/10.5543/tkda.2015.56805
Kjaergaard AD, Johansen JS, Bojesen SE, Nordestgaard BG (2016) Role of inflammatory marker YKL-40 in the diagnosis, prognosis and cause of cardiovascular and liver diseases. Crit Rev Clin Lab Sci 53(6):396–408. https://doi.org/10.1080/10408363.2016.1190683
Mygind ND, Harutyunyan MJ, Mathiasen AB, Ripa RS, Thune JJ, Gotze JP, Johansen JS, Kastrup J (2011) The influence of statin treatment on the inflammatory biomarkers YKL-40 and HsCRP in patients with stable coronary artery disease. Inflamm Res 60(3):281–287. https://doi.org/10.1007/s00011-010-0266-5
Bonneh-Barkay D, Bissel SJ, Kofler J, Starkey A, Wang G, Wiley CA (2012) Astrocyte and macrophage regulation of YKL-40 expression and cellular response in neuroinflammation. Brain pathology (Zurich, Switzerland) 22(4):530–546. https://doi.org/10.1111/j.1750-3639.2011.00550.x
Kanneganti M, Kamba A, Mizoguchi E (2012) Role of chitotriosidase (chitinase 1) under normal and disease conditions. J Epithelial Biol Pharmacol 5:1–9
Lungato L, Tamanaha P, Rodrigues MDB, Visniauskas B, Chagas JR, Tufik S, D’Almeida V (2013) Chitotriosidase as a marker of macrophage activation after paradoxical sleep deprivation. Sleep science (Sao Paulo, Brazil) 6(1):5–8
Tamanaha P, D'Almeida V, Calegare BF, Tomita LY, Bittencourt LR, Tufik S (2013) 24 bp duplication of CHIT1 gene and determinants of human chitotriosidase activity among participants of EPISONO, a population-based cross-sectional study, Sao Paulo, Brazil. Clin Biochem 46(12):1084–1088. https://doi.org/10.1016/j.clinbiochem.2013.05.044
Alanbay I, Ercan CM, Sakinci M, Coksuer H, Ozturk M, Tapan S (2012) A macrophage activation marker chitotriosidase in women with PCOS: does low-grade chronic inflammation in PCOS relate to PCOS itself or obesity? Arch Gynecol Obstet 286(4):1065–1071. https://doi.org/10.1007/s00404-012-2425-0
Bjornsdottir E, Benediktsdottir B, Pack AI, Arnardottir ES, Kuna ST, Gislason T, Keenan BT, Maislin G, Sigurdsson JF (2016) The prevalence of depression among untreated obstructive sleep apnea patients using a standardized psychiatric interview. J Clin Sleep Med 12(1):105–112. https://doi.org/10.5664/jcsm.5406
Arnardottir ES, Verbraecken J, Goncalves M, Gjerstad MD, Grote L, Puertas FJ, Mihaicuta S, McNicholas WT, Parrino L (2016) Variability in recording and scoring of respiratory events during sleep in Europe: a need for uniform standards. J Sleep Res 25(2):144–157. https://doi.org/10.1111/jsr.12353
Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, Troester MT, Vaughn BV (2017) AASM scoring manual updates for 2017 (version 2.4). J Clin Sleep Med 13(5):665–666. https://doi.org/10.5664/jcsm.6576
Xu L, Han F, Keenan BT, Kneeland-Szanto E, Yan H, Dong X, Chang Y, Zhao L, Zhang X, Li J, Pack AI, Kuna ST (2017) Validation of the Nox-T3 portable monitor for diagnosis of obstructive sleep apnea in Chinese adults. J Clin Sleep Med 13(5):675–683. https://doi.org/10.5664/jcsm.6582
Cairns A, Wickwire E, Schaefer E, Nyanjom D (2014) A pilot validation study for the NOX T3(TM) portable monitor for the detection of OSA. Sleep & breathing = Schlaf & Atmung 18(3):609–614. https://doi.org/10.1007/s11325-013-0924-2
Iber C, Medicine AAoS (2007) The AASM manual for the scoring of sleep and associated events: Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine,
Hollak CE, van Weely S, van Oers MH, Aerts JM (1994) Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J Clin Invest 93 (3):1288–1292. doi:https://doi.org/10.1172/jci117084
Kyrgios I, Galli-Tsinopoulou A, Stylianou C, Papakonstantinou E, Arvanitidou M, Haidich AB (2012) Elevated circulating levels of the serum acute-phase protein YKL-40 (chitinase 3-like protein 1) are a marker of obesity and insulin resistance in prepubertal children. Metabolism 61(4):562–568. https://doi.org/10.1016/j.metabol.2011.09.004
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328(17):1230–1235. https://doi.org/10.1056/nejm199304293281704
Shinohara E, Kihara S, Yamashita S, Yamane M, Nishida M, Arai T, Kotani K, Nakamura T, Takemura K, Matsuzawa Y (1997) Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Intern Med 241(1):11–18
Grunstein R, Wilcox I, Yang TS, Gould Y, Hedner J (1993) Snoring and sleep apnoea in men: association with central obesity and hypertension. Int J Obes Relat Metab Disord 17(9):533–540
Vincent HK, Taylor AG (2006) Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (2005) 30(3):400–418. https://doi.org/10.1038/sj.ijo.0803177
Clement K, Langin D (2007) Regulation of inflammation-related genes in human adipose tissue. J Intern Med 262(4):422–430. https://doi.org/10.1111/j.1365-2796.2007.01851.x
Lavie L (2003) Obstructive sleep apnoea syndrome--an oxidative stress disorder. Sleep Med Rev 7(1):35–51
Tasali E, Ip MS (2008) Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc 5(2):207–217. https://doi.org/10.1513/pats.200708-139MG
Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R, Bouloumie A (2006) Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 49(4):744–747. https://doi.org/10.1007/s00125-006-0173-z
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112(12):1796–1808. https://doi.org/10.1172/jci19246
Hajer GR, van Haeften TW, Visseren FL (2008) Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 29(24):2959–2971. https://doi.org/10.1093/eurheartj/ehn387
Iwata T, Kuwajima M, Sukeno A, Ishimaru N, Hayashi Y, Wabitsch M, Mizusawa N, Itakura M, Yoshimoto K (2009) YKL-40 secreted from adipose tissue inhibits degradation of type I collagen. Biochem Biophys Res Commun 388(3):511–516. https://doi.org/10.1016/j.bbrc.2009.08.024
Majde JA, Krueger JM (2005) Links between the innate immune system and sleep. J Allergy Clin Immunol 116(6):1188–1198. https://doi.org/10.1016/j.jaci.2005.08.005
Benca RM, Quintas J (1997) Sleep and host defenses: a review. Sleep 20(11):1027–1037
Johansen JS, Hvolris J, Hansen M, Backer V, Lorenzen I, Price PA (1996) Serum YKL-40 levels in healthy children and adults. Comparison with serum and synovial fluid levels of YKL-40 in patients with osteoarthritis or trauma of the knee joint. Br J Rheumatol 35(6):553–559
Funding
This work was supported by the National Institutes of Health grant HL94307 for “Endophenotypes of Sleep Apnea and Role of Obesity,” the Icelandic Research fund grant 173701-052, the Eimskip Fund of the University of Iceland, and the Landspitali University Hospital Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E.S. Arnardottir is a part-time consultant for Nox Medical and has received honarium from Weinmann and ResMed, outside the scope of the submitted work. Other authors declare no conflict of interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
(PDF 423 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Teitsdottir, U.D., Arnardottir, E.S., Bjornsdottir, E. et al. Obesity modulates the association between sleep apnea treatment and CHI3L1 levels but not CHIT1 activity in moderate to severe OSA: an observational study. Sleep Breath 22, 1101–1109 (2018). https://doi.org/10.1007/s11325-018-1731-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-018-1731-6