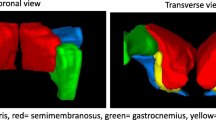
Abstract
Purpose
To compare targeted imaging of vascular endothelial growth factor (VEGF) receptors vs. αvβ3 integrins in a mouse hindlimb ischemia model of peripheral artery disease.
Procedures
Male wild-type (WT) C57BL/6 mice (8- to 10-week old) (n = 24) underwent left femoral artery ligation. The right leg served as control. Five days later, mice were injected with either VEGF receptor targeting [99mTc]DOTA-PEG-scVEGF ([99mTc]scV) (n = 8) or with αvβ3-targeting tracer [99mTc]HYNIC-cycloRGD ([99mTc]RGD) (n = 8) and underwent single photon emission computed tomography (SPECT) x-ray computed tomography imaging. To assess non-specific [99mTc]scV uptake, six additional mice received a mixture of [99mTc]scV and 30-fold excess of targeting protein, scVEGF. Tracer uptake as %ID was measured using volumetric regions encompassing the hindlimb muscles and as %ID/g from harvested limb muscles. Double and triple immunofluorescent analysis on tissue sections established localization of αvβ3, VEGFR-1, VEGFR-2, as well as certain cell lineage markers.
Results
Tracer uptake, as %ID/g, was higher in ligated limbs of mice injected with [99mTc]scV compared to ligated hindlimbs in mice injected with [99mTc]RGD (p = 0.02). The ratio of tracer uptake for ligated/control hindlimb was borderline higher for [99mTc]scV than for [99mTc]RGD (p = 0.06). Immunofluorescent analysis showed higher prevalence of VEGFR-1, VEGFR-2, and αvβ3, in damaged vs. undamaged hindlimb tissue, but with little co-localization of these markers. Double immunofluorescent staining showed partial co-localization of VEGFR-1, VEGFR-2, and αvβ3, with endothelial cell marker FVIII, but not with CD31. Immunostaining for VEGFR-1 and VEGFR-2 additionally co-localized with lineage markers for endothelial progenitor cell and monocytes/macrophages, with a more diverse pattern of co-localization for VEGFR-2.
Conclusion
In a mouse hindlimb ischemia model of peripheral artery disease, [99mTc]scV SPECT tracer-targeting VEGF receptors showed a more robust signal than [99mTc]RGD tracer-targeting αvβ3. Immunofluorescent analysis suggests that uptake of [99mTc]scV and [99mTc]RGD in damaged tissue is due to non-overlapping cell populations and reflects different dynamic processes and that enhanced uptake of [99mTc]scV may be due to the presence of VEGF receptors on additional cell types.






Similar content being viewed by others
References
Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FGR, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RAG, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME (2017) AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary—a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 135:e686–e725
Norgren L, Hiatt WR, Dormandy JA et al (2007) TASC II working group. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 45(Suppl):S1–S68
Cooke JP, Losordo DW (2015) Modulating the vascular response to limb ischemia: angiogenic and cell therapies. Circ Res 116:1561–1578
Madeddu P, Emanueli C, Spillmann F, Meloni M, Bouby N, Richer C, Alhenc-Gelas F, van Weel V, Eefting D, Quax PHA, Hu Y, Xu Q, Hemdahl AL, van Golde J, Huijberts M, de Lussanet Q, Boudier HS, Couffinhal T, Duplaa C, Chimenti S, Staszewsky L, Latini R, Baumans V, Levy BI (2006) Murine models of myocardial and limb ischemia: diagnostic end-points and relevance to clinical problems. Vasc Pharmacol 45:281–301
Huveneers S, Truong H, Danen HJ (2007) Integrins: signaling, disease, and therapy. Int J Radiat Biol 83:743–751
Sun CC, Qu XJ, Gao ZH (2016) Arginine-glycine-aspartate-binding integrins as therapeutic and diagnostic targets. Am J Ther 23:e198–e207
Hua J, Dobrucki LW, Sadeghi MM, Zhang J, Bourke BN, Cavaliere P, Song J, Chow C, Jahanshad N, van Royen N, Buschmann I, Madri JA, Mendizabal M, Sinusas AJ (2005) Noninvasive imaging of angiogenesis with a 99mTc-labeled peptide targeted at αvβ3 integrin after murine hindlimb ischemia. Circulation 111:3255–3260
Lee KH, Jung KH, Song SH, Kim DH, Lee BC, Sung HJ, Han YM, Choe YS, Chi DY, Kim BT (2005) Radiolabeled RGD uptake and αv integrin expression is enhanced in ischemic murine hindlimbs. J Nucl Med 46:472–478
Jeong JM, Hong MK, Chang YS, Lee YS, Kim YJ, Cheon GJ, Lee DS, Chung JK, Lee MC (2008) Preparation of a promising angiogenesis PET imaging agent: [68Ga]-labeled c(RGDyK)-isothiocyanatobenzyl-1,4,7-triazacyclononane-1,4,7-triacetic acid and feasibility studies in mice. J Nucl Med 49:830–836
Dobrucki LW, Dione DP, Kalinowski L, Dione D, Mendizabal M, Yu J, Papademetris X, Sessa WC, Sinusas AJ (2009) Serial noninvasive targeted imaging of peripheral angiogenesis: validation and application of a semiautomated quantitative approach. J Nucl Med 50:1356–1363
Faintuch BL, Teodoro R, Oliveira EA et al (2011) Neovascularization after ischemic injury: evaluation with [99m]Tc-HYNIC-RGD. Acta Cir Bras 26:58–63
Tekabe Y, Shen X, Luma J et al (2011) Imaging the effect of receptor for advanced glycation endproducts on angiogenic response to hindlimb ischemia in diabetes. EJNMMI Res 1:3. https://doi.org/10.1186/2191-219X-1-3
Tekabe Y, Anthony T, Li Q, Ray R, Rai V, Zhang G, Schmidt AM, Johnson LL (2015) Treatment effect with anti-RAGE F(ab')2 antibody improves hindlimb angiogenesis and blood flow in Type 1 diabetic mice with left femoral artery ligation. Vasc Med 20:212–218
Shibuya M (2013) Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 153:13–19
Ganta VC, Choi M, Kutateladze A, Annex BH (2017) VEGF165b modulates endothelial VEGFR1-STAT3 signaling pathway and angiogenesis in human and experimental peripheral arterial disease. Circ Res 120:282–295
Giles AJ, Reid CM, Evans JD et al (2016) Activation of hematopoietic stem/progenitor cells promotes immunosuppression within the pre–metastatic niche. Cancer Res 76:1335–1347
Wu FT, Stefanini MO, Mac Gabhann F, Kontos CD, Annex BH, Popel AS (2010) A systems biology perspective on sVEGFR1: its biological function, pathogenic role and therapeutic use. J Cell Mol Med 14:528–552
Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, Detmar M (1996) Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the αvβ3 integrin, osteopontin, and thrombin. Am J Pathol 149:293–305
Wu J, Strawn TL, Luo M (2015) Plasminogen activator inhibitor-1 inhibits angiogenic signaling by uncoupling vascular endothelial growth factor receptor-2-αVβ3 integrin cross talk. Arterioscler Thromb Vasc Biol 35:111–120
Lu E, Wagner WR, Schellenberger U, Abraham JA, Klibanov AL, Woulfe SR, Csikari MM, Fischer D, Schreiner GF, Brandenburger GH, Villanueva FS (2003) Targeted in vivo labeling of receptors for vascular endothelial growth factor: approach to identification of ischemic tissue. Circulation 108:97–103
Willmann JK, Chen K, Wang H, Paulmurugan R, Rollins M, Cai W, Wang DS, Chen IY, Gheysens O, Rodriguez-Porcel M, Chen X, Gambhir SS (2008) Monitoring of the biological response to murine hindlimb ischemia with 64Cu-labeled vascular endothelial growth factor-121 positron emission tomography. Circulation 117:915–922
Hamada Y, Gonda K, Takeda M et al (2011) In vivo imaging of the molecular distribution of the VEGF receptor during angiogenesis in a mouse model of ischemia. Blood 118:e93–e100
Backer MV, Levashova Z, Patel V, Jehning BT, Claffey K, Blankenberg FG, Backer JM (2007) Molecular imaging of VEGF receptors in angiogenic vasculature with single-chain VEGF-based probes. Nat Med 13:504–509
Levashova Z, Backer M, Backer JM, Blankenberg FG (2008) Direct site-specific labeling of the Cys-tag moiety in scVEGF with technetium 99m. Bioconjug Chem 19:1049–1054
Tekabe Y, Kollaros M, Zerihoun A, Zhang G, Backer MV, Backer JM, Johnson LL (2014) Imaging VEGF receptor expression to identify accelerated atherosclerosis. EJNMMI Res 4:41–49
Tekabe Y, Johnson L, Rodriquez K et al (2017) Selective imaging of vascular endothelial growth factor receptor-1 and receptor-2 in atherosclerotic lesions in diabetic and non-diabetic ApoE−/− mice. Mol Imaging Biol 20:85–93
Decristoforo C, Faintuch-Linkowski B, Rey A, von Guggenberg E, Rupprich M, Hernandez-Gonzales I, Rodrigo T, Haubner R (2006) [99mTc]Hynic-RGD for imaging integrin avb3 expression. Nucl Med Biol 33:945–952
Rissanen TT, Vajanto I, Hiltunen MO et al (2002) Expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 (KDR/Flk-1) in ischemic skeletal muscle and its regeneration. Am J Pathol 16:1393–1403
Miraliakbari R, Francalancia NA, Lust RM, Gerardo JA, Ng PC, Sun YS, Chitwood WR Jr (2000) Differences in myocardial and peripheral VEGF and KDR levels after acute ischemia. Ann Thorac Surg 69:1750–1754
Germani A, Di Carlo A, Mangoni A (2003) Vascular endothelial growth factor modulates skeletal myoblast function. Am J Pathol 163:1417–1428
Turner NA, Moake JL (2015) Factor VIII is synthesized in human endothelial cells, packaged in Weibel-Palade bodies and secreted bound to ULVWF strings. PLoS One 10:e0140740
Lertkiatmongkol P, Liao D, Mei H, Hu Y, Newman PJ (2016) Endothelial functions of platelet/endothelial cell adhesion molecule-1 (CD31). Curr Opin Hematol 23:253–259
Walenta K, Friedrich EB, Sehnert F, Werner N, Nickenig G (2005) In vitro differentiation characteristics of cultured human mononuclear cells-implications for endothelial progenitor cell biology. Biochem Biophys Res Commun 333:476–482
Sangidorj O, Yang SH, Jang HR, Lee JP, Cha RH, Kim SM, Lim CS, Kim YS (2010) Bone marrow-derived endothelial progenitor cells confer renal protection in a murine chronic renal failure model. Am J Physiol Renal Physiol 299:F325–F335
Imoukhuede PI, Dokun AO, Annex BH, Popel AS (2013) Endothelial cell-by-cell profiling reveals the temporal dynamics of VEGFR1 and VEGFR2 membrane localization after murine hindlimb ischemia. Am J Physiol Heart Circ Physiol 304:H1085–H1093
Meyer J-P, Edwards KJ, Kozlowski P, Backer MV, Backer JM, Lewis JS (2016) Selective imaging of VEGFR-1 and VEGFR-2 receptors using [89Zr]-labeled single-chain VEGF mutants. J Nucl Med 57:1811–1816
Amano H, Kato S, Ito Y, Eshima K, Ogawa F, Takahashi R, Sekiguchi K, Tamaki H, Sakagami H, Shibuya M, Majima M (2015) The role of vascular endothelial growth factor receptor-1 signaling in the recovery from ischemia. PLoS One 10:e0131445
Ikutomi M, Sahara M, Nakajima T, Minami Y, Morita T, Hirata Y, Komuro I, Nakamura F, Sata M (2015) Diverse contribution of bone marrow-derived late-outgrowth endothelial progenitor cells to vascular repair under pulmonary arterial hypertension and arterial neointimal formation. J Mol Cell Cardiol 86:121–135
Urbich C, Dimmeler S (2004) Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 8:343–353
Kopp HG, Ramos CA, Rafii S (2006) Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr Opin Hematol 13:175–181
Patschan D, Kribben A, Muller GA (2016) Postischemic microvasculopathy and endothelial progenitor cell-based therapy in ischemic AKI: update and perspectives. Am J Physiol Renal Physiol 311:F382–F394
Fu SS, Li FJ, Wang YY, You AB, Qie YL, Meng X, Li JR, Li BC, Zhang Y, da Li Q (2013) Kallikrein gene-modified EPCs induce angiogenesis in rats with ischemic hindlimb and correlate with integrin alphavbeta3 expression. PLoS One 8:e73035
Tepekoylu C, Wang FS, Kozaryn R et al (2013) Shock wave treatment induces angiogenesis and mobilizes endogenous CD31/CD34-positive endothelial cells in a hindlimb ischemia model: implications for angiogenesis and vasculogenesis. J Thorac Cardiovasc Surg 146:971–978
Guerin CL, Loyer X, Vilar J, Cras A, Mirault T, Gaussem P, Silvestre JS, Smadja DM (2015) Bone-marrow-derived very small embryonic-like stem cells in patients with critical leg ischaemia: evidence of vasculogenic potential. Thromb Haemost 113:1084–1094
Alev C, Ii M, Asahara T (2011) Endothelial progenitor cells: a novel tool for the therapy of ischemic diseases. Antioxid Redox Signal 15:949–965
Adini A, Adini I, Ghosh K, Benny O, Pravda E, Hu R, Luyindula D, D’Amato RJ (2013) The stem cell marker prominin-1/CD133 interacts with vascular endothelial growth factor and potentiates its action. Angiogenesis 16:405–416
Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S (2000) Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95:952–958
Kuhlmann MT, Klocke R, Nikol S (2007) Therapeutic angiogenesis for peripheral artery disease: cytokine therapy. Vasa 36:253–260
Ryu J, Lee CW, Hong KH, Shin JA, Lim SH, Park CS, Shim J, Nam KB, Choi KJ, Kim YH, Han KH (2008) Activation of fractalkine/CX3CR1 by vascular endothelial cells induces angiogenesis through VEGF-A/KDR and reverses hindlimb ischaemia. Cardiovasc Res 78:333–340
Cochain C, Rodero MP, Vilar J, Recalde A, Richart AL, Loinard C, Zouggari Y, Guerin C, Duriez M, Combadiere B, Poupel L, Levy BI, Mallat Z, Combadiere C, Silvestre JS (2010) Regulation of monocyte subset systemic levels by distinct chemokine receptors controls post-ischaemic neovascularization. Cardiovasc Res 88:186–195
van den Hengel LG, Hellingman AA, Nossent AY, van Oeveren-Rietdijk AM, de Vries MR, Spek CA, van Zonneveld AJ, Reitsma PH, Hamming JF, de Boer HC, Versteeg HH, Quax PHA (2013) Protease-activated receptor (PAR)2, but not PAR1, is involved in collateral formation and anti-inflammatory monocyte polarization in a mouse hindlimb ischemia model. PLoS One 8:e61923
Seaman SA, Cao Y, Campbell CA, Peirce SM (2016) Macrophage recruitment and polarization during collateral vessel remodeling in murine adipose tissue. Microcirculation 23:75–87
Ruan J, Hyjek E, Kermani P, Christos PJ, Hooper AT, Coleman M, Hempstead B, Leonard JP, Chadburn A, Rafii S (2006) Magnitude of stromal hemangiogenesis correlates with histologic subtype of non-Hodgkin's lymphoma. Clin Cancer Res 12:5622–5631
Zhuang PY, Shen J, Zhu XD, Lu L, Wang L, Tang ZY, Sun HC (2013) Prognostic roles of cross-talk between peritumoral hepatocytes and stromal cells in hepatocellular carcinoma involving peritumoral VEGF-C, VEGFR-1 and VEGFR-3. PLoS One 8:e64598
Financial Support
5 P01 HL060901-12 (Schmidt, PI) NIH/NHLBI, Title: RAGE and mechanisms of Vascular Dysfunction.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 662 kb)
Rights and permissions
About this article
Cite this article
Tekabe, Y., Li, Q., Zhang, G. et al. Imaging VEGF Receptors and αvβ3 Integrins in a Mouse Hindlimb Ischemia Model of Peripheral Arterial Disease. Mol Imaging Biol 20, 963–972 (2018). https://doi.org/10.1007/s11307-018-1191-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-018-1191-1