Abstract
Purpose
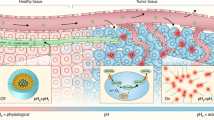
Acidification of extracellular space promotes tumor development, progression, and invasiveness. pH (low) insertion peptides (pHLIP® peptides) belong to the class of pH-sensitive membrane peptides, which target acidic tumors and deliver imaging and/or therapeutic agents to cancer cells within tumors.
Procedures
Ex vivo fluorescent imaging of tissue and organs collected at various time points after administration of different pHLIP® variants conjugated with fluorescent dyes of various polarity was performed. Methods of multivariate statistical analyses were employed to establish classification between fluorescently labeled pHLIP® variants in multidimensional space of spectral parameters.
Results
The fluorescently labeled pHLIP® variants were classified based on their biodistribution profile and ability of targeting of primary tumors. Also, submillimeter-sized metastatic lesions in lungs were identified by ex vivo imaging after intravenous administration of fluorescent pHLIP® peptide.
Conclusions
Different cargo molecules conjugated with pHLIP® peptides can alter biodistribution and tumor targeting. The obtained knowledge is essential for the design of novel pHLIP®-based diagnostic and therapeutic agents targeting primary tumors and metastatic lesions.






Similar content being viewed by others
References
Damaghi M, Wojtkowiak JW, Gillies RJ (2013) pH sensing and regulation in cancer. Front Physiol 4:370
Gatenby RA, Gawlinski ET, Gmitro AF et al (2006) Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res 66:5216–23
Gatenby RA, Smallbone K, Maini PK et al (2007) Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer. Br J Cancer 97:646–53
Hashim AI, Zhang X, Wojtkowiak JW et al (2011) Imaging pH and metastasis. NMR Biomed 24:582–91
Chiche J, Brahimi-Horn MC, Pouyssegur J (2010) Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J Cell Mol Med 14:771–94
Parks SK, Chiche J, Pouyssegur J (2013) Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer 13:611–23
Andreev OA, Engelman DM, Reshetnyak YK (2010) pH-sensitive membrane peptides (pHLIPs) as a novel class of delivery agents. Mol Membr Biol 27:341–52
Andreev OA, Engelman DM, Reshetnyak YK (2014) Targeting diseased tissues by pHLIP insertion at low cell surface pH. Front Physiol 5:97
Andreev OA, Engelman DM, Reshetnyak YK (2009) Targeting acidic diseased tissue: new technology based on use of the pH (low) insertion peptide (pHLIP). Chim Oggi 27:34–7
Weerakkody D, Moshnikova A, Thakur MS et al (2013) Family of pH (low) insertion peptides for tumor targeting. Proc Natl Acad Sci U S A 110:5834–9
Karabadzhak AG, Weerakkody D, Wijesinghe D et al (2012) Modulation of the pHLIP transmembrane helix insertion pathway. Biophys J 102:1846–55
Andreev OA, Karabadzhak AG, Weerakkody D et al (2010) pH (low) insertion peptide (pHLIP) inserts across a lipid bilayer as a helix and exits by a different path. Proc Natl Acad Sci U S A 107:4081–6
Reshetnyak YK, Segala M, Andreev OA, Engelman DM (2007) A monomeric membrane peptide that lives in three worlds: in solution, attached to, and inserted across lipid bilayers. Biophys J 93:2363–72
Reshetnyak YK, Andreev OA, Segala M et al (2008) Energetics of peptide (pHLIP) binding to and folding across a lipid bilayer membrane. Proc Natl Acad Sci U S A 105:15340–5
Vavere AL, Biddlecombe GB, Spees WM et al (2009) A novel technology for the imaging of acidic prostate tumors by positron emission tomography. Cancer Res 69:4510–6
Karabadzhak AG, An M, Yao L et al (2014) pHLIP-FIRE, a cell insertion-triggered fluorescent probe for imaging tumors demonstrates targeted cargo delivery in vivo. ACS Chem Biol 9:2545–53
Daumar P, Wanger-Baumann CA, Pillarsetty N et al (2012) Efficient (18)F-labeling of large 37-amino-acid pHLIP peptide analogues and their biological evaluation. Bioconjug Chem 23:1557–66
Cheng CJ, Bahal R, Babar IA et al (2015) MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature 518:107–10
Cruz-Monserrate Z, Roland CL, Deng D et al (2014) Targeting pancreatic ductal adenocarcinoma acidic microenvironment. Sci Rep 4:4410
Adochite RC, Moshnikova A, Carlin SD et al (2014) Targeting breast tumors with pH (low) insertion peptides. Mol Pharm 11:2896–905
Tapmeier TT, Moshnikova A, Beech J et al (2015) The pH low insertion peptide pHLIP Variant 3 as a novel marker of acidic malignant lesions. Proc Natl Acad Sci U S A 112:9710–5
Reshetnyak YK, Yao L, Zheng S et al (2011) Measuring tumor aggressiveness and targeting metastatic lesions with fluorescent pHLIP. Mol Imaging Biol 13:1146–56
Loja MN, Luo Z, Greg Farwell D et al (2013) Optical molecular imaging detects changes in extracellular pH with the development of head and neck cancer. Int J Cancer 132:1613–23
Luo Z, Loja MN, Farwell DG et al (2014) Widefield optical imaging of changes in uptake of glucose and tissue extracellular pH in head and neck cancer. Cancer Prev Res (Phila) 7:1035–44
Macholl S, Morrison MS, Iveson P et al (2012) In vivo pH imaging with (99m)Tc-pHLIP. Mol Imaging Biol 14:725–34
Viola-Villegas NT, Carlin SD, Ackerstaff E et al (2014) Understanding the pharmacological properties of a metabolic PET tracer in prostate cancer. Proc Natl Acad Sci U S A 111:7254–9
Fox J, Weisberg S (2011) An R companion to applied regression. Sage, Thousand Oaks
Tao K, Fang M, Alroy J, Sahagian GG (2008) Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer 8:228
Yang J, Mani SA, Donaher JL et al (2004) Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117:927–39
Eckhardt BL, Parker BS, van Laar RK et al (2005) Genomic analysis of a spontaneous model of breast cancer metastasis to bone reveals a role for the extracellular matrix. Mol Cancer Res 3:1–13
Serganova I, Rizwan A, Ni X et al (2011) Metabolic imaging: a link between lactate dehydrogenase A, lactate, and tumor phenotype. Clin Cancer Res 17:6250–61
Ward JH Jr (1963) Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58:236–44
Pulaski BA, Ostrand-Rosenberg S (2001) Mouse 4T1 breast tumor model. Curr Protoc Immunol Chapter 20:Unit 20 22.
Yang S, Zhang JJ, Huang XY (2012) Mouse models for tumor metastasis. Methods Mol Biol 928:221–8
Keereweer S, Van Driel PB, Snoeks TJ et al (2013) Optical image-guided cancer surgery: challenges and limitations. Clin Cancer Res 19:3745–54
Keereweer S, Kerrebijn JD, van Driel PB et al (2011) Optical image-guided surgery—where do we stand? Mol Imaging Biol 13:199–207
Frangioni JV (2003) In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol 7:626–34
Acknowledgments
This work was supported by the National Institute of General Medical Sciences Grant RO1 GM073857 to OAA and YKR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal studies were conducted according to the animal protocol AN04-12-011 approved by the Institutional Animal Care and Use Committee at the University of Rhode Island, in compliance with the principles and procedures outlined by NIH for the Care and Use of Animals.
Conflict of Interest
Andreev and Reshetnyak own stocks in company pHLIP, Inc. They have disclosed those interestsfully to the University of Rhode Island, and have in place an approved plan for managing any potentialconflicts. The following authors, Adochite, Moshnikova, Golijanin, and Katenka, declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1278 kb)
Rights and permissions
About this article
Cite this article
Adochite, RC., Moshnikova, A., Golijanin, J. et al. Comparative Study of Tumor Targeting and Biodistribution of pH (Low) Insertion Peptides (pHLIP® Peptides) Conjugated with Different Fluorescent Dyes. Mol Imaging Biol 18, 686–696 (2016). https://doi.org/10.1007/s11307-016-0949-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-016-0949-6