Abstract
Purpose
The aim of the present study was to evaluate the use of 2-deoxy-2-[18F]fluoro-d-glucose ([18F]FDG) as a noninvasive strategy to assess the time course of inflammatory processes after inhalation of ZnO nanoparticles (NPs) in rats.
Procedures
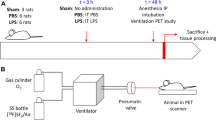
Healthy, male Sprague–Dawley rats (n = 30) were divided in two groups of 15 animals each. Animals from one group (n = 15) were submitted to ZnO NPs inhalation in a chamber (10 nm to 4 μm particle size; maximum in number concentration, ∼200 nm; concentration = 245 mg/m3). Animals from the other group (n = 15, sham group) were also exposed following the same procedure, but no NPs were introduced into the chamber. Six animals per group were submitted to [18F]FDG-positron emission tomography (PET) studies at days 1, 7, and 28 after exposition, and the [18F]FDG influx constant (K i ) for the lungs was calculated using Patlak graphical analysis and an image derived blood input function. Nine animals per group were killed at 1, 7 and 28 days after exposure (n = 3 per group and time point), and the lungs were harvested and submitted to immunohistochemical and histological analysis.
Results
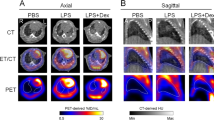
Significantly higher mean whole-lung K i values were obtained for animals exposed to NPs at days 1 and 7 after exposure (0.0045 ± 0.0016 min−1 and 0.0047 ± 0.0015 min−1, respectively) compared to controls (0.0024 ± 0.0010 min−1 and 0.0019 ± 0.0011 min−1 at 1 and 7 days, respectively). The K i value for exposed animals dropped to 0.0023 ± 0.0010 min−1 at day 28. This value was not significantly different from the values obtained at 1, 7, and 28 days for the control group. Immunofluorescence staining on lung tissue slices from animals exposed to ZnO NPs showed an increase in CD11b reactivity at days 1 and 7, followed by a decrease in CD11b positive cells at 28 days. Hematoxylin–eosin staining showed histological alterations in the exposed lungs to ZnO NPs at days 1 and 7 that recovered at 28 days postexposure.
Conclusions
The [18F]FDG influx rate constant (K i ) could be determined by PET using Patlak analysis and a corrected image derived input function. Higher K i values were obtained for animals exposed to ZnO NPs at days 1 and 7 after exposition. These results were in good concordance with immunohistochemical assays performed on harvested tissue samples.






Similar content being viewed by others
References
Blasco C, Picó Y (2011) Determining nanomaterials in food. TrAC 30:84–99
Zielecka M, Bujnowska E, Kepska B et al (2011) Antimicrobial additives for architectural paints and impregnates. Prog Org Coat 72:193–201
Blyszko J, Kiernozycki W, Guskos N et al (2008) Study of mechanical properties of concrete with low concentration of magnetic nanoparticles. J Non-Cryst Solids 354:4515–4518
Stroyuk AL, Shvalagin VV, Kuchmii SY (2005) Photochemical synthesis and optical properties of binary and ternary metal–semiconductor composites based on zinc oxide nanoparticles. J Photochem Photobio A 173:185–194
Wang MC (2010) Synthesis of zinc oxide nanocrystalline powders for cosmetic applications. Ceram Int 36:693–698
Yukuphanoglu F (2011) Synthesis and electro-optic properties of nanosized-boron doped cadmium oxide thin films for solar cell applications. Sol Energy 85:2704–2709
Sahay R, Sundaramurthy J, Suresh KP et al (2012) Synthesis and characterization of CuO nanofibers, and investigation for its suitability as blocking layer in ZnO nps based dye sensitized solar cell and as photocatalyst in organic dye degradation. J Solid State Chem 186:261–267
Locatelli E, Gil L, Israel LL et al (2012) Biocompatible nanocomposite for PET/MRI hybrid imaging. Int J Nanomed 7:6021–6033
Weiss C, Diabate S (2011) A special issue on nanotoxicology. Arch toxicol 85:705–706
Ju-Nam Y, Lead JR (2008) Manufactured nanoparticles: an overview of their chemistry, interactions and potential environmental implications. Sci Total Environ 400:396–414
Handy RD, von der Kammer F, Lead JR et al (2008) The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 17:287–314
Osmond MJ, McCall MJ (2010) Zinc oxide nanoparticles in modern sunscreens: an analysis of potential exposure and hazard. Nanotoxicology 4:15–41
Sayes CM, Reed KL, Wahrheit DB (2007) Assessing toxicity of fine and nanoparticles: comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol Sci 97:163–180
Cho WS, Duffin R, Poland CA et al (2012) Differential pro-inflammatory effects of metal oxide nanoparticles and their soluble ions in vitro and in vivo; zinc and copper nanoparticles, but not their ions, recruit eosinophils to the lungs. Nanotoxicology 6:22–35
Locke LW, Williams MB, Fairchild KD et al (2011) FDG-PET quantification of lung inflammation with image-derived blood input function in mice. Int J Mol Imaging 22:356730
Chen DL, Bedient TJ, Kozlowski J et al (2009) [18F]Fluorodeoxyglucose positron emission tomography for lung antiinflammatory response evaluation. Am J Respir Crit Care Med 180:533–539
Martin TR (2010) Neutrophils and lung injury: getting it right. J Clin Invest 110:1603–1605
Zambelli V, Di Grigoli G, Scanziani M et al (2012) Time course of metabolic activity and cellular infiltration in a murine model of acid-induced lung injury. Intens Care Med 38:694–701
Chen DL, Schuster DP (2004) Positron emission tomography with [18F]fluorodeoxyglucose to evaluate neutrophil kinetics during acute lung injury. Am J Physiol Lung Cell Mol Physiol 286:L834–L840
Jones HA, Clark RJ, Rhodes CG et al (1994) In vivo measurement of neutrophil activity in experimental lung inflammation. Am J Resp Crit Care 149:1635–1639
Jones HA, Sriskandan S, Peters AM et al (1997) Dissociation of neutrophil emigration and metabolic activity in lobar pneumonia and bronchiectasis. Eur Respir J 10:795–803
Schroeder T, Vidal-Melo MF, Musch G et al (2008) Modeling pulmonary kinetics of 2-deoxy-2-[18F]fluoro-D-glucose during acute lung injury. Acad Radiol 15:763–775
Zhou Z, Kozlowski J, Goodrich AL et al (2005) Molecular imaging of lung glucose uptake after endotoxin in mice. Am J Physiol Lung Cell Mol Physiol 289:L760–L768
Hansson L, Ohlsson T, Valind S et al (1999) Glucose utilization in the lungs of septic rats. Eur J Nucl Med 26:1340–1344
Fang YH, Muzic RF Jr (2008) Spillover and partial-volume correction for image-derived input functions for small-animal 18F-FDG PET studies. J Nucl Med 49:606–614
van der Weerdt AP, Klein LJ, Boellaard R et al (2001) Image-derived input functions for determination of MRGlu in cardiac 18F-FDG PET scans. J Nucl Med 42:1622–1629
Patlak CS, Blasberg RG, Fenstermacher JD (1987) Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 3:1–7
Clausen SK, Bergqvist M, Poulsen LK et al (2003) Development of sensitisation or tolerance following repeated OVA inhalation in BALB/cJ mice. Dose-dependency and modulation by the Al(OH)3 adjuvant. Toxicology 184:51–68
Acknowledgments
The authors would like to thank the 7th Framework Program for funding this work through the HINAMOX PROJECT contract agreement no. NMP4-SL-2009-228825, and QNANO SP4-Capacities-2010-262163 for support. The authors also gratefully acknowledge the support of the Ministerio de Economía y Competitividad (former Ministerio de Ciencia e Innovación) for financial support (CENIT CIN/1559/2009), Professor Ronald F. Ziolo from Centro de Investigación en Química Aplicada (Saltillo, Mexico) for fruitful discussion and Plasmachem GmbH for kindly donating ZnO NPs.
Conflict of Interest
None of the authors have any conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 106 kb)
Rights and permissions
About this article
Cite this article
Pérez-Campaña, C., Gómez-Vallejo, V., Puigivila, M. et al. Assessing Lung Inflammation After Nanoparticle Inhalation Using 2-deoxy-2-[18F]fluoro-d-glucose Positron Emission Tomography Imaging. Mol Imaging Biol 16, 264–273 (2014). https://doi.org/10.1007/s11307-013-0682-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-013-0682-3