Abstract
Purpose
The bacterial gene MagA imparts magnetic properties to mammalian cells and provides a basis for cell tracking by magnetic resonance imaging (MRI). In a mouse model of tumor growth from transplanted cells, we used repetitive MRI to demonstrate the in vivo imaging potential of MagA expression relative to a modified ferritin overexpression system, lacking regulation through iron response elements (HF + LF).
Procedures
Subcutaneous tumor xenografts were monitored weekly from days 2 to 34 post-injection. Small animal MRI employed balanced steady-state free precession. Imaging was correlated with tumor histology using hematoxylin, Prussian Blue, Ki-67, and BS-1 lectin.
Results
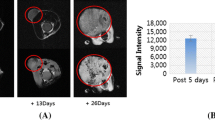
Tumor heterogeneity with respect to tissue morphology and magnetic resonance (MR) contrast was apparent within a week of cell transplantation. In MagA- and HF + LF-expressing tumors, MR contrast enhancement was recorded up to day 20 post-injection and 0.073-cm3 tumor volumes. MagA-expressing tumors showed increases in both quantity and quality of MR contrast as measured by fractional void volume and contrast-to-noise ratio, respectively. MR contrast in both MagA- and HF + LF-expressing tumors was maximal by day 13, doubling fractional void volume 1 week ahead of controls.
Conclusions
MagA- and HF + LF-expressing tumor xenografts augment MR contrast after 1 week of growth. MagA expression increases MR contrast within days of cell transplantation and provides MR contrast comparable to HF + LF. MagA has utility for monitoring cell growth and differentiation, with potential for in vivo detection of reporter gene expression using MRI.






Similar content being viewed by others
References
Foulkes W, Reis-Filho J, Narod S (2010) Tumor size and survival in breast cancer—a reappraisal. Nat Rev Clin Oncol 7:348–353
Roukos D, Ziogas D, Katsios C (2010) Multigene assays and isolated tumor cells for early breast cancer treatment: time for bionetworks. Expert Rev Anticancer Ther 10:1187–1195
Schnitt S (2010) Classification and prognosis of invasive breast cancer: from morphology to molecular taxonomy. Mod Pathol 23:S60–S64
Brader P, Serganova I, Blasberg R (2013) Noninvasive molecular imaging using reporter genes. J Nucl Med 54:167–172
Croker A, Goodale D, Chu J et al (2009) High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J Cell Mol Med 13:2236–2252
Mees C, Nemunaitis J, Senzer N (2009) Transcription factors: their potential as targets for an individualized therapeutic approach to cancer. Cancer Gene Ther 16:103–112
Feig S (2010) Cost-effectiveness of mammography, MRI, and ultrasonography for breast cancer screening. Radiol Clin North Am 48:879–891
Meng Y, Ward S, Cooper K et al (2011) Cost-effectiveness of MRI and PET imaging for the evaluation of axillary lymph node metastases in early stage breast cancer. Eur J Surg Oncol 37:40–46
Taneja C, Edelsberg J, Weycker D et al (2009) Cost effectiveness of breast cancer screening with contrast-enhanced MRI in high-risk women. J Am Coll Radiol 6:171–179
Budde M, Frank J (2009) Magnetic tagging of therapeutic cells for MRI. J Nucl Med 50:171–174
Gilad A, Ziv K, McMahon M et al (2008) MRI reporter genes. J Nucl Med 49:1905–1908
Goldhawk D, Rohani R, Sengupta A et al (2012) Using the magnetosome to model effective gene-based contrast for magnetic resonance imaging. WIRES Nanomed Nanobiotechnol 4:378–388
Goldhawk D, Lemaire C, McCreary C et al (2009) Magnetic resonance imaging of cells overexpressing MagA, an endogenous contrast agent for live cell imaging. Mol Imaging 8:129–139
Zurkiya O, Chan AW, Hu X (2008) MagA is sufficient for producing magnetic nanoparticles in mammalian cells, making it an MRI reporter. Magn Reson Med 59:1225–1231
Lefevre C, Menguy N, Abreu F et al (2011) A cultured greigite-producing magnetotactic bacterium in a novel group of sulfate-reducing bacteria. Science 334:1720–1723
Nishida K, Silver P (2012) Induction of biogenic magnetization and redox control by a component of the target of rapamycin complex 1 signaling pathway. PLoS Biol 10:e1001269
Hsu C-Y, Chan Y-P (2011) Identification and localization of proteins associated with biomineralization in the iron deposition vesicles of honeybees (Apis mellifera). PLoS One 6:e19088
Diebel C, Proksch R, Green C et al (2000) Magnetite defined a vertebrate magnetoreceptor. Nature 406:299–302
Treiber C, Salzer M, Riegler J et al (2012) Clusters of iron-rich cells in the upper beak of pigeons are macrophages not magnetosensitive neurons. Nature 484:367–370. doi:10.1038/nature11046
Cohen B, Ziv K, Plaks V et al (2009) Ferritin nanoparticles as magnetic resonance reporter gene. Wiley Interdiscip Rev Nanomed Nanobiotechnol 1:181–188
Cohen B, Ziv K, Plaks V et al (2007) MRI detection of transcriptional regulation of gene expression in transgenic mice. Nat Med 13:498–503
Deans AE, Wadghiri YZ, Bernas LM et al (2006) Cellular MRI contrast via coexpression of transferrin receptor and ferritin. Magn Reson Med 56:51–59
Genove G, DeMarco U, Xu H et al (2005) A new transgene reporter for in vivo magnetic resonance imaging. Nat Med 11:450–454
Heyn C, Ronald J, MacKenzie L et al (2006) In vivo magnetic resonance imaging of single cells in mouse brain with optical validation. Magn Reson Med 55:23–29
Ramadan S, Heyn C, MacKenzie L et al (2008) Ex-vivo cellular MRI with b-SSFP: quantitative benefits of 3T over 1.5T. Magn Reson Mater Phys Biol Med 21:251–259
Tomayko MM, Reynolds CP (1989) Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol 24:148–154
Gonzalez-Lara LE, Xu X, Hofstetrova K et al (2011) The use of cellular magnetic resonance imaging to track the fate of iron-labeled multipotent stromal cells after direct transplantation in a mouse model of spinal cord injury. Mol Imaging Biol 13:702–711
de Chickera S, Willert C, Mallet C et al (2012) Cellular MRI as a suitable, sensitive non-invasive modality for correlating in vivo migratory efficiencies of different dendritic cell populations with subsequent immunological outcomes. Int Immunol 24:29–41
Rohani R, de Chickera SN, Willert C et al (2011) In vivo cellular MRI of dendritic cell migration using micrometer-sized iron oxide (MPIO) particles. Mol Imaging Biol 13:679–694
Chamson-Reig A, Arany E, Hill D (2010) Lineage tracing and resulting phenotype of haemopoietic-derived cells in the pancreas during beta cell regeneration. Diabetologia 53:2188–2197
Nicholson M, Arany E, Hill D (2010) Changes in islet microvasculature following streptozotocin-induced b-cell loss and subsequent replacement in the neonatal rat. Exp Biol Med 235:189–198
Li J, Yang L, Song L et al (2009) Astrocyte elevated gene-1 is a proliferation promoter in breast cancer via suppressing transcriptional factor FOXO1. Oncogene 28:3188–3196
McGirr R, Ejbick CE, Carter DE et al (2005) Glucose dependence of the regulated secretory pathway in alphaTC1-6 cells. Endocrinology 146:4514–4523
Liu J, Cheng EC, Long RC et al (2009) Noninvasive monitoring of embryonic stem cells in vivo with MRI transgene reporter. Tissue Eng Part C Methods 15:739–747
Kim HS, Cho HR, Choi SH et al (2010) In vivo imaging of tumor transduced with bimodal lentiviral vector encoding human ferritin and green fluorescent protein on a 1.5T clinical magnetic resonance scanner. Cancer Res 70:7315–7324
Aung W, Hasegawa S, Koshikawa-Yano M et al (2009) Visualization of in vivo electroporation-mediated transgene expression in experimental tumors by optical and magnetic resonance imaging. Gene Ther 16:830–839
Mallett CL, Foster PJ (2011) Optimization of the balanced steady state free precession (bSSFP) pulse sequence for magnetic resonance imaging of the mouse prostate at 3T. PLoS One 6:e18361
Mallett CL, McFadden C, Chen Y, Foster PJ (2012) Migration of iron-labeled KHYG-1 natural killer cells to subcutaneous tumors in nude mice, as detected by magnetic resonance imaging. Cytotherapy 14:743–751
Sengupta A, Rohani R, Thompson R, et al (2012) Transverse and longitudinal MRI relaxation rates in MagA-expressing cancer cells. Dublin, Ireland. Abstract P345
Nakamura C, Burgess JG, Sode K, Matsunaga T (1995) An iron-regulated gene, magA, encoding an iron transport protein of Magnetospirillum sp. strain AMB-1. J Biol Chem 270:28392–28396
Nakamura C, Kikuchi T, Burgess JG, Matsunaga T (1995) Iron-regulated expression and membrane localization of the MagA protein in Magnetospirillum sp. strain AMB-1. J Biochem 118:23–27
Uebe R, Henn V, Schuler D (2012) The MagA protein of Magnetospirilla is not involved in bacterial magnetite biomineralization. J Bacteriol 194:1018–1023
Farrar W (ed) (2009) Cancer stem cells. Cambridge University Press, New York
Ishimori T, Tatsumi M, Wahl RL (2005) Tumor response assessment is more robust with sequential CT scanning than external caliper measurements. Acad Radiol 12:776–781
Jensen MM, Jorgensen JT, Binderup T, Kjaer A (2008) Tumor volume in subcutaneous mouse xenografts measured by microCT is more accurate and reproducible than determined by 18F-FDG-microPET or external caliper. BMC Med Imaging 8:16
Acknowledgments
The authors thank Aaron Chapman, Becky McGirr, and Alice Wang for technical assistance. Tom Crohns performed the cryosectioning. This research was partially funded by grants from the Cancer Imaging Network of Ontario, supported by Cancer Care Ontario, and the Lawson Internal Research Fund. DEG, RTT, and FSP are supported by the Ontario Research Fund Research Excellence project 02-038, Imaging for Cardiovascular Therapeutics: myocardial cell therapy for heart failure.
Conflicts of interest
Drs. Goldhawk, Prato, and Thompson are authors on a patent describing the use of magnetosome genes in eukaryotic cells.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 366 kb)
Rights and permissions
About this article
Cite this article
Rohani, R., Figueredo, R., Bureau, Y. et al. Imaging Tumor Growth Non-invasively Using Expression of MagA or Modified Ferritin Subunits to Augment Intracellular Contrast for Repetitive MRI. Mol Imaging Biol 16, 63–73 (2014). https://doi.org/10.1007/s11307-013-0661-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-013-0661-8