Abstract
Purpose
The suboptimal features of the spectral properties of the leading fluorescence resonance energy transfer (FRET) pair, cyan fluorescent protein (CFP)/yellow fluorescent protein (YFP), limit the full promise of FRET imaging. To overcome the drawbacks, we developed FRET-based, intra-molecular biosensors consisting of CFP/discomona sp red fluorescent protein (DsRed) or green fluorescent protein (GFP)/DsRed as donor/acceptor fluorophores.
Procedures
The biosensors were expressed in NIH3T3 cells. In vitro fluorescence spectroscopy and Rho GTPase activation assays were used to confirm that Rac1 or Cdc42 was activated in serum-starved cells following stimulation with insulin or bradykinin. The transient changes of the amount, location, and translocation of activated Rac1 or Cdc42 in living cells were tracked with confocal imaging.
Results
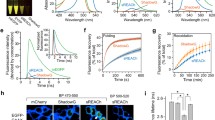
The increase of FRET efficiency was achieved in the cells expressing the biosensors and was proportional to the levels of activated Rac1 or Cdc42. The localized, transitional, and transient FRET signals were directly and quantitatively imaged with high spatial and temporal resolution. The biosensors were used to analyze and judge the GEF or GAP activities of putative regulatory proteins for Rac1 or Cdc42.
Conclusion
DsRed is a more suitable acceptor in FRET pair with CFP than with GFP in terms of the spectral overlap between the donor and acceptor. The approach can also be applied to many other types of protein behavior in living cells.







Similar content being viewed by others
Abbreviations
- FRET:
-
Fluorescence resonance energy transfer
- CFP:
-
Cyan fluorescent protein
- GFP:
-
Green fluorescent protein
- YFP:
-
Yellow fluorescent protein
- GEF:
-
Guanine nucleotide exchange factors
- GAP:
-
GTPase-activating proteins
- PAK1:
-
p21-activated kinase1
- N-WASP:
-
Neural Wiskott-Aldrich syndrome protein
- GBD:
-
GTPase binding domain
References
Van Aelst L, D'Souza-Schorey C (1997) Rho GTPases and signaling networks. Genes Dev 11:2295–2322
Etienne-Manneville S, Hall A (2002) Rho GTPases in cell biology. Nature 420:629–635
Jaffe A, Hall A (2005) Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21:247–269
Govek E, Newey S, Van Aelst L (2005) The role of the Rho GTPases in neuronal development. Genes Dev 19:1–49
Ellenbroek S, Collard J (2007) Rho GTPases: functions and association with cancer. Clin Exp Metastasis 24:657–672
Sahai E, Marshall C (2002) Rho GTPases and cancer. Nat Rev Cancer 2:133–142
Itoh RE, Kurokawa K, OhbaY YH, Mochizuki N, Matsuda M (2002) Activation of Rac and Cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol Cell Biol 22:6582–6591
Lorenz M, Yamaguchi H, Wang Y, Singer RH, Condeelis J (2004) Imaging sites of N-WASP activity in lamellipodia and invadopodia of carcinoma cells. Curr Biol 14:697–703
Kurokawa K, Nakamura T, Aoki K, Matsuda M (2005) Mechanism and role of localized activation of Rho-family GTPases in growth factor-stimulated fibroblasts and neuronal cells. Biochem Soc Transact 33:631–634
Centonze V, Sun M, Masuda A, Gerritsen H, Herman B (2003) Fluorescence resonance energy transfer imaging microscopy. Methods Enzymol 360:542–560
Berney C, Danuser G (2003) FRET or No FRET: a quantitative comparison. Biophys J 84:3992–4010
Miyawaki A (2003) Visualization of the spatial and temporal dynamics of intracellular signaling. Dev Cell 4:295–305
Jares-Erijman E, Jovin T (2003) FRET imaging. Nat Biotechnol 21:1387–1395.
Meyer T, Teruel M (2003) Fluorescence imaging of signaling networks. Trend Cell Biol 13:101–106
Day R (2005) Imaging protein behavior inside the living cell. Mol Cell Endocrinol 230:1–6
Yasuda R (2006) Imaging spatiotemporal dynamics of neuronal signaling using fluorescence resonance energy transfer and fluorescence lifetime imaging microscopy. Curr Opin Neurobiol 6:551–561
Wang Y, Shyy J, Chien S (2008) Fluorescence proteins, live-cell imaging, and mechanobiology: seeing is believing. Annu Rev Biomed Eng 15:1–38
Erickson M, Moon D, Yue D (2003) DsRed as a potential FRET partner with CFP and GFP. Biophys J 85:599–611
Piston D, Kremers G (2007) Fluorescent protein FRET: the good, the bad, and the ugly. Trends Biochem Sci 32:407–414
Ai H, Hazelwood K, Davidson M, Campbell R (2008) Fluorescent protein FRET pairs for ratiometric imaging of dual biosensors. Nat Methods 5:401–403
Baird G, Zacharias D, Tsien R (2000) Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. Proc Natl Acad Sci USA 97:11984–11989
Mizuno H, Sawano A, Eli P, Hama H, Miyawaki A (2001) Red fluorescent protein from Discosoma as a fusion tag and a partner for fluorescence resonance energy transfer. Biochem 40:2502–2510
Kraynov VS, Chamberlain C, Bokoch GMM, Schwartz A, Slabaugh S, Hahn KM (2000) Localized Rac activation dynamics visualized in living cells. Science 290:333–337
Prehoda K, Scott E, Mullins R, Lim W (2000) Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science 290:801–806
Krogt G, Ogink J, Ponsioen B, Jalink K (2008) A comparison of donor-acceptor Pairs for genetically encoded FRET sensors: application to the Epac cAMP sensor as an example. PLoS ONE 3:e1916
Van Engelenburg S, Palmer A (2008) Fluorescent biosensors of protein function. Curr Opin Chem Biol 12:60–65
Zal T, Gascoigne N (2004) Using live FRET imaging to reveal early protein–protein interactions during T cell activation. Curr Opin Immunol 16:674–683
Zhang J, Campbell R, Ting A, Tsien R (2002) Creating new fluorescent probes for cell biology. Nature/Reviews/Mol Cell Biol 3:906–916
Galperin E, Verkhusha V, Sorkin A (2004) Three-chromophore FRET microscopy to analyze multiprotein interactions in living cells. Nat Methods 3:209–217
Shaner N, Lin M, McKeown M, Steinbach P, Hazelwood K, Davidson M, Tsien R (2008) Improving the photostability of bright monomeric orange and red fluorescent proteins. Nat Methods 5:545–551
Rossman K, Der C, Sondek J (2005) GEF means go: turning on Rho GTPases with guanine nucleotide –exchange factors. Nat Rev Mol Cell Biol 6:167–180
Guo X, Stafford LJ, Bryan B, Xia C, Ma W, Wu X, Liu D, Songyang Z, Liu M (2003) A Rac/Cdc42 specific exchange factor, GEFT, induces cell proliferation, transformation and migration. J Biol Chem 278:13207–13215
Nalbant P, Hodgson L, Kraynov V, Toutchkine A, Hahn KM (2004) Activation of endogenous Cdc42 visualized in living cells. Science 305:1615–1619
Schmidt A, Hall A (2002) Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev 16:1587–1609
Shaner N, Steinbach P, Tsien R (2005) A guide to choosing fluorescent proteins. Nat Methods 2:905–909
Alieva NO, Konzen KA, Field SF, Meleshkevitch EA, Hunt ME, Beltran-Ramirez V, Miller DJ, Wiedenmann J, Salih A, Matz MV (2008) Diversity and evolution of coral fluorescent proteins. PLoS ONE 3:e2680
Acknowledgements
We thank Dr. Chunyi Tong for the help in assays of fluorescence spectroscopy and Mr. Ju Peng for the work in confocal microscopy. This work was supported by the grants from National Natural Science Foundation of China (30470887, 30871269), Hunan Provincial Natural Science Foundation of China (98JJY2004), and Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry of China.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Significance:
This manuscript describes the FRET-based intra-molecular biosensors using DsRed as an acceptor in FRET pair with CFP or GFP that can be used to image the induced activation of Rac1, Cdc42 in living cells, and to analyze and judge the GEF or GAP activities of putative regulatory proteins for Rac1 or Cdc42.
Rushi Liu and Daoquan Ren contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Fluorescence emission spectra analysis in cell lysates expressing the biosensors of GFP/DsRed upon excitation at 488 nm after stimulation for 5 min. Two emission peaks at 488 nm (CFP) and 583 nm (DsRed) were observed (JPEG 50 kb)
Fig. S2
FRET measurements for two individual cells depicted the changing fluorescence intensity of FRET signals, representing the changing Rac1 activation levels in vivo in process of the induced activation (JPEG 53 kb)
Fig. S3
FRET measurements for two individual cells revealed the changes of Cdc42 activation levels in vivo in process of the induced activation (JPEG 46 kb)
(MPG 3812 kb)
(MPG 5592 kb)
Rights and permissions
About this article
Cite this article
Liu, R., Ren, D., Liu, Y. et al. Biosensors of DsRed as FRET Partner with CFP or GFP for Quantitatively Imaging Induced Activation of Rac, Cdc42 in Living Cells. Mol Imaging Biol 13, 424–431 (2011). https://doi.org/10.1007/s11307-010-0381-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-010-0381-2