Abstract
Purpose
Recent data have indicated that 68Ga-DOTA-NOC positron emission tomography/X-ray computed tomography (PET/CT) may yield improved images in a shorter acquisition protocol than 111In-DTPA-octreotide (OctreoScan®, OCT). Therefore, we performed a prospective comparison of 68Ga-DOTA-NOC and OCT for the detection of neuroendocrine tumors (NETs).
Methods
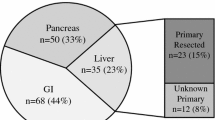
Nineteen patients (eight carcinoid, nine pancreatic NETs, and two NE carcinoma of unknown origin) with previous positive OCT scans underwent 68Ga-DOTA-NOC PET/CT and OCT single-photon emission computed tomography imaging for staging or follow-up. Findings were compared by region and verified with conventional imaging.
Results
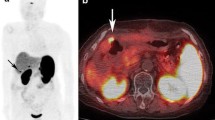
All images of both modalities demonstrated focal uptake, often at multiple sites. 68Ga-DOTA-NOC images were clearer than OCT images, facilitating interpretation. Similar foci were identified with both modalities in 41 regions, with additional foci on 68Ga-DOTA-NOC in 21 and on OCT in 15 regions. CT, magnetic resonance imaging, or ultrasound confirmed the concordant findings in 31 of 41 regions and findings seen with 68Ga-DOTA-NOC only in 15 of 21 regions. Findings seen with OCT only were less clear and were only confirmed in 4 of 15 regions. 68Ga-DOTA-NOC had impact on staging in four patients and on management in three patients.
Conclusions
Although 68Ga-DOTA-NOC and OCT images were similar, in this study, 68Ga-DOTA-NOC demonstrated more true positive tumor foci and was better tolerated by patients. This direct comparison supports replacement of OCT with 68Ga-DOTA-NOC-PET/CT in the evaluation of NETs.





Similar content being viewed by others
References
Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP, Moss SF, Nilsson O, Rindi G, Salazar R, Ruszniewski P, Sundin A (2008) Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 9:61–72
Oberg K, Eriksson B (2005) Nuclear medicine in the detection, staging and treatment of gastrointestinal carcinoid tumours. Best Pract Res Clin Endocrinol Metab 19:265–276
Kwekkeboom DJ, Krenning EP, de Jong M (2000) Peptide receptor imaging and therapy. J Nucl Med 41:1704–1713
Kaltsas G, Rockall A, Papadogias D, Reznek R, Grossman AB (2004) Recent advances in radiological and radionuclide imaging and therapy of neuroendocrine tumours. Eur J Endocrinol 151:15–27
Froidevaux S, Eberle AN, Christe M, Sumanovski L, Heppeler A, Schmitt JS, Eisenwiener K, Beglinger C, Mäcke HR (2002) Neuroendocrine tumor targeting: study of novel gallium-labeled somatostatin radiopeptides in a rat pancreatic tumor model. Int J Cancer 98:930–937
Antunes P, Ginj M, Zhang H, Waser B, Baum RP, Reubi JC, Maecke H (2007) Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging 34:982–993
Wild D, Schmitt JS, Ginj M, Mäcke HR, Bernard BF, Krenning E, De Jong M, Wenger S, Reubi JC (2003) DOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labelling with various radiometals. Eur J Nucl Med Mol Imaging 30:1338–1347
Buchmann I, Henze M, Engelbrecht S, Eisenhut M, Runz A, Schäfer M, Schilling T, Haufe S, Herrmann T, Haberkorn U (2007) Comparison of 68Ga-DOTATOC and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging 34:1617–1626
Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, Kovacs P, Von Guggenberg E, Bale R, Virgolini IJ (2007) 68Ga-DOTA-Tyr3-Octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med 48:508–518
Gabriel M, Oberauer A, Dobrozemsky G, Decristoforo C, Putzer D, Kendler D, Uprimny C, Kovacs P, Bale R, Virgolini IJ (2009) 68Ga-DOTA-Tyr3-Octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. J Nucl Med 50:1427–1434
Win Z, Al-Nahhas A, Towey D, Todd JF, Rubello D, Lewington V, Gishen P (2007) 68Ga-DOTATATE PET in neuroectodermal tumours: first experience. Nucl Med Commun 28:359–363
Wild D, Mäcke HR, Waser B, Reubi JC, Ginj M, Rasch H, Müller-Brand J, Hofmann M (2005) 68Ga-DOTANOC: a first compound for PET imaging with high affinity for somatostatin receptor subtypes 2 and 5. Eur J Nucl Med Mol Imaging 32:724
Pettinato C, Sarnelli A, Di Donna M, Civollani S, Nanni C, Montini G, Di Pierro D, Ferrari M, Marengo M, Bergamini C (2008) 68Ga-DOTANOC: biodistribution and dosimetry in patients affected by neuroendocrine tumors. Eur J Nucl Med Mol Imaging 35:72–79
Kwekkeboom DJ, Kooij PP, Bakker WH, Macke HR, Krenning EP (1999) Comparison of 111In-DOTA-Tyr3-octreotide and 111In-DTPA-octreotide in the same patients: biodistribution, kinetics, organ and tumor uptake. J Nucl Med 40:762–767
Baum R, Niesen A, Leonhardi J, Wortmann R, Mueller D, Roesch F (2005) Receptor PET/CT imaging of neuroendocrine tumours using the Ga-68 labelled, high affinity somatostatin analogue DOTA-1-Nal3 Octreotide (DOTA-NOC): clinical results in 327 patients. Eur J Nucl Med Mol Imaging 32(Suppl 1):54–55S (Abstract)
Ambrosini V, Castellucci P, Rubello D, Nanni C, Musto A, Allegri V, Montini GC, Mattioli S, Grassetto G, Al-Nahhas A, Franchi R, Fanti S (2009) 68Ga-DOTA-NOC: a new PET tracer for evaluating patients with bronchial carcinoid. Nucl Med Commun 30:281–286
Ambrosini V, Tomassetti P, Castellucci P, Campana D, Montini G, Rubello D, Nanni C, Rizzello A, Franchi R, Fanti S (2008) Comparison between 68Ga-DOTA-NOC and 18F-DOPA PET for the detection of gastro-entero-pancreatic and lung neuro-endocrine tumours. Eur J Nucl Med Mol Imaging 35:1431–1438
Fanti S, Ambrosini V, Tomassetti P, Castellucci P, Montini G, Allegri V, Grassetto G, Rubello D, Nanni C, Franchi R (2008) Evaluation of unusual neuroendocrine tumours by means of 68Ga-DOTA-NOC PET. Biomed Pharmacother 62:667–671
Prasad V, Ambrosini V, Hommann M, Hoersch D, Fanti S, Baum RP (2010) Detection of unknown primary neuroendocrine tumours (CUP-NET) using 68Ga-DOTA-NOC receptor PET/CT. Eur J Nucl Med Mol Imaging 37:67–77
Zasadny KR, Wahl RL (1993) Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-d-glucose: variations with body weight and a method for correction. Radiology 189:847–850
Wehrmann C, Senftleben S, Zachert C, Müller D, Baum RP (2007) Results of individual patient dosimetry in peptide receptor radionuclide therapy with 177Lu DOTA-TATE and 177Lu DOTA-NOC. Cancer Biother Radiopharm 22:406–416
Zhernosekov KP, Filosofov DV, Baum RP, Aschoff P, Bihl H, Razbash AA, Jahn M, Jennewein M, Rösch F (2007) Processing of generator-produced 68Ga for medical application. J Nucl Med 48:1741–1748
Acknowledgments
We gratefully acknowledge the help of Dganit Barak and Ella Shamalove in assisting with patient preparation and organization. We acknowledge the role of Desideriu Laky of the Cyclotron/Radiochemistry Unit, Hadassah Hebrew University Hospital, Jerusalem, in the development of the tracer synthesis. We also acknowledge the contribution of Dani Vaknin and Avihai Y Bross of the Radiochemistry Department, Nahal Soreq NRC, Yavne, and Kobi Ben-Meir, Roni Michael of the Cyclotron Department, Nahal Soreq NRC, Yavne, for their part in organizing and participating in the synthesis of 68Ga-DOTA-NOC.
Conflict of Interest Disclosure
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript’s significance: Our small prospective study investigates the clinical relevance of differences between imaging NETs with OctreoScan and the newer PET tracer, DOTA-NOC, with its improved patient tolerability, reduced machine and personnel time, and cost. Although the advantages of the new tracer have been described by others, systematic comparison is important to assess the differences between the two imaging methods before switching to DOTA-NOC.
Yodphat Krausz and Nanette Freedman contributed equally to this work.
Appendix: 68Ga-DOTA-NOC Synthesis
Appendix: 68Ga-DOTA-NOC Synthesis
68Ga-DOTA-NOC was prepared according to the procedure described by Zhernosekov et al. [22]. The radiochemical transformation was fully automated using the GE F-18 fluorination Tracerlab module with major hardware and software modifications. To the GE module, an electric Valco valve with 14 channels was added and connected to ten reagent vials and to a nitrogen gas supply valve. This electric Valco valve was connected by its exit to a three-way valve, which was further connected to a cation exchange column. The latter three-way valve was also connected via a series of valves to the 68Ge/68Ga generator (1,125–1,875 MBq, Obninsk Inc. Russia). The 68Ga was eluted with 5–7 ml 0.1 N HC1 (Ultrapure). The solution was passed through the cation exchanger column (Biorad 1X8, donated by Dr. Frank Roesch, Germany). In this manner, 99% of the activity eluted from the generator was trapped by the cation exchange column. Then, the column was washed with 1 ml, 80% acetone/20% 0.15 N HCl solution for the removal of 68Ge and other metal impurities. In the next step, 0.2 ml of the eluting solution (97.6% acetone/2.4% 0.05 N HCl) was loaded onto the column and left for 2.5 min; then, the 68Ga was eluted from the column using an additional 0.2 ml of the same eluting solution. In this manner, approx. 90% of the activity was recovered and transferred to the reactor which contained the starting material (DOTA-NOC [1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid]-1-NaI3-Octreotide acetate salt, 50 μg, GMP grade, ABX Germany) dissolved in 7.6 ml of HEPES buffer (pH 4.1). The peptide solution was pre-heated under vacuum for 1 min at approx 30°C prior to the addition of the solution of 68Ga, which was added drop-wise. Then, this solution was further heated to 98°C for an additional 8 min. The final pH of the reaction solution was 3.7. After cooling the solution to 30°C, the reaction mixture was loaded by nitrogen gas pressure onto RP C-18 (STRATA) column, which was further washed toward a waste vial by 5 ml of water (WFI). The pure 68Ga-DOTA-NOC was eluted from the column using 0.4 ml ethanol and 8.5 ml saline solution. Finally, the product solution was filtered via 0.22-μm GV sterile filter and collected in the product vial. During the process, the 68Ga activity was monitored by built-in radioactive detectors. The 68Ge content in both the generator eluent and in the final product was periodically measured by gamma spectroscopy using HPGE detector. The 68Ge eluted with the 68Ga was always in accordance with the manufacturer analysis certificate, while its presence in the final product was under the detectable limit. The chemical and radiochemical purity of the final product was determined by ITLC and HPLC: Method 1: ITLC-SG 60, mobile phase 0.1 M sodium citrate, pH 5, 68Ga-DOTA-NOC Rf = 0, free Ga-68 Rf = 0.9. Method 2: ITLC-SG 60 mobile phase 1 M ammonium acetate/methanol (1:1) 68Ga-DOTA-NOC Rf = 0.8–1, colloid Ga Rf = 0–0.2 HPLC; C18 column 250 × 4 mm; flow rate of 1 ml/min using a gradient solution. The gradient was gradually changed during the first 20 min from initial 20% acetonitrile/80% water +0.01% TFA to 40% acetonitrile/60% water + 0.01% TFA and following the end of the procedure was reversed gradually back to the original 20% acetonitrile/80% water +0.01% TFA, 68Ga-DOTA-NOC Rt = 18 min, and free 68Ga Rt = 2 min. The ethanol and acetone content in the final product were determined by GC. The acetone content was <0.5% and the ethanol was <5%. The average synthesis time including the generator eluting time (n = 40) was 23 min. The average radiochemical decay-corrected yield was 54 ± 4.74%, and the non-decay-corrected yield was 70 ± 3%. Radiochemical and chemical purity were >95%.
Initially, when working with low doses of activity, not more than approximately 500 MBq, we performed the synthesis in water (WFI), with a final pH of 2.2. However, when increasing the activity level to above 500 MBq, additional radioactive peaks were detected in the HPLC with retention times between 14 and 17 min. The calculated area percentage of these labeled by-products was dependent on the starting radioactivity levels, and it ranged between 10% and 35%. When the process was done in HEPES buffer, these impurities were eliminated and the purity of the final obtained product was >95%. The nature of these labeled by-products is unclear. We assume that when working with high activity doses, degradation of the starting material ensues. It seems that the HEPES molecules have a radio-protective effect. The stability of the product was monitored by ITLC and HPLC, 2.5 h post-EOS. There was no significant change in the purity of the 68Ga DOTA-NOC, and this permitted successful production.
Rights and permissions
About this article
Cite this article
Krausz, Y., Freedman, N., Rubinstein, R. et al. 68Ga-DOTA-NOC PET/CT Imaging of Neuroendocrine Tumors: Comparison with 111In-DTPA-Octreotide (OctreoScan®). Mol Imaging Biol 13, 583–593 (2011). https://doi.org/10.1007/s11307-010-0374-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-010-0374-1