Abstract
Purpose
Carbon-11 labeled N-butan-2-yl-1-(2-chlorophenyl)-N-methylisoquinoline-3-carboxamide ([11C]PK11195) is a peripheral benzodiazepine receptor (PBR) antagonist that is used as a positron emission tomography (PET) radiopharmaceutical for neuroinflammatory imaging. This study was designed to investigate the radiation dosimetry of [11C]PK11195.
Procedures
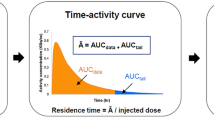
Whole-body distribution kinetics of intravenously administered [11C]PK11195 in rats was assessed by means of dynamic PET imaging, and estimates for human radiation dosimetry were calculated. Rat plasma and various tissue homogenates obtained at different time points after intravenous injection of [11C]PK11195 were analyzed by reversed-phase gradient radio-HPLC method using online radiodetection. In addition, in vitro stability of [11C]PK11195 was determined in rat brain homogenate by incubation at +37°C.
Results
PET imaging of rats showed the highest radioactivity levels in heart, kidneys, thyroid gland, liver, and lungs. The radioactivity cleared rapidly from lungs and slowly from heart and liver. However, much of the radioactivity retained in kidneys, which was in concordance with the observed low urinary excretion of [11C]PK11195. Extrapolating from the rat data, the effective dose of [11C]PK11195 for a 70-kg man was estimated to be 4.2 ± 0.3 µSv/MBq. Five different radiometabolites were detected in rat plasma, and the level of intact [11C]PK11195 decreased from 80% ± 11% (mean ± SD) at 10 min to 44% ± 5% at 40 min after injection. In rat heart, brain, kidney, and lung homogenates, more than 90% of total radioactivity originated from intact [11C]PK11195. In liver, however, the amount of [11C]PK11195 was approximately 70% and decreased over time, indicating metabolism by liver enzymes.
Conclusions
[11C]PK11195 showed a fast uptake in many rat tissues and it was metabolized relatively fast in vivo, but not in brain in vitro. The estimated effective dose for humans speaks for the use of [11C]PK11195 in human PET imaging.






Similar content being viewed by others
References
Papadopoulos V, Baraldi M, Guilarte TR et al (2006) Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 27:402–409
Casellas P, Galiegue S, Basile AS (2002) Peripheral benzodiazepine receptors and mitochondrial function. Neurochem Int 40:475–486
Krueger KE (1995) Molecular and functional properties of mitochondrial benzodiazepine receptors. Biochim Biophys Acta 241:453–470
Skrabanek L, Campagne F (2001) TissueInfo: high-throughput identification of tissue expression profiles and specificity. Nucleic Acids Res 29:E102
Huminiecki L, Lloyd AT, Wolfe KH (2003) Congruence of tissue expression profiles from Gene Expression Atlas. SAGEmap and TissueInfo databases. BMC Genomics 4:31
Junck L, Olson JM, Ciliax BJ et al (1989) PET imaging of human gliomas with ligands for the peripheral benzodiazepine binding site. Ann Neurol 26:752–758
Vowinckel E, Reutens D, Becher B et al (1997) PK11195 binding to the peripheral benzodiazepine receptor as a marker of microglia activation in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurosci Res 50:345–353
Banati RB, Newcombe J, Gunn RN et al (2000) The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain 123:2321–2337
Turner MR, Cagnin A, Turkheimer FE et al (2004) Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis 15:601–609
Ouchi Y, Yoshikawa E, Sekine Y et al (2005) Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann Neurol 57:168–175
Pappata S, Levasseur M, Gunn RN et al (2000) Thalamic microglial activation in ischemic stroke detected in vivo by PET and [11C]PK1195. Neurology 55:1052–1054
De Vos F, Dumont F, Santens P, Slegers G, Dierckx R, De Reuck J (1999) High-performance liquid chromatographic determination of [11C]-1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinoline carboxamid in mouse plasma and tissue and in human plasma. J Chromatography B736:61–66
Charbonneau P, Syrota A, Crouzel C, Valois J-M, Prenant C, Crouzel M (1986) Peripheral-type benzodiazepine receptors in the living heart characterized by positron emission tomography. Circulation 73:476–483
Pike VW, Halldin C, Crouzel C et al (1993) Radioligands for PET studies of central benzodiazepine receptors and PK (peripheral benzodiazepine) binding sites - current status. Nucl Med Biol 20:503–525
Luthra SK, Osman S, Turton DR, Vaja V, Dowsett K, Brady F (1993) An automated system based on solid phase extraction and HPLC for the routine determination in plasma of unchanged [11C]-L-deprenyl; [11C]diprenorphine; [11C]flumazenil; [11C]raclopride; and [11C]Scherring 23390. J Labelled Compds Radiopharm 32:518–520
Greuter HNJM, van Ophemert PLB, Luurtsema G et al (2005) Optimizing an online SPE–HPLC method for analysis of (R)-[11C]1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide [(R)-[11C]-PK11195] and its metabolites in humans. Nucl Med Biol 32:307–312
Debruyne JC, Versijpt J, Van Laere KJ et al (2003) PET visualization of microglia in multiple sclerosis patients using [11C]-PK11195. Eur J Neurol 10:257–264
Kirschner A, Ice R, Beierwaltes W (1975) Radiation dosimetry of 131I-19-iodocholesterol: the pitfalls of using tissue concentration data, the author’s reply. J Nucl Med 16:248–249
Rhodes CG, Wollmer P, Fazio F, Jones T (1981) Quantitative measurement of regional extravascular lung density using positron emission and transmission tomography. J Comput Assist Tomogr 5:783–791
Davies B, Morris T (1993) physiological parameters in laboratory animals and humans. Pharm Res 10:1093–1095
Stabin MG, Sparks RB, Crowe E (2005) OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 46:1023–1027
Anholt RR, De Souza EB, Oster-Granite ML, Snyder SH (1985) Peripheral-type benzodiazepine receptors: autoradiographic localization in whole-body sections of neonatal rats. J Pharmacol Exp Ther 233:517–526
Addendum 6 to ICRP Publication 53: Radiation doses of radiopharmaceuticals 2002. Available at: www.icrp.org/docs/Add_5-7_to_P53.pdf.
Fujimura Y, Kreisl WC, Zoghbi SS, et al (2008) Quantification of specific binding in brain and radiation dosimetry of [11C](R)-PK11195 in rhesus monkey. NeuroImage 41:T112
Swahn CG, Halldin C, Farde L (1992) HPLC-method for determination of ligand metabolism during PET-studies. Ann Univ Turkuensis D88:A65-A66
Roivainen A, Någren K, Hirvonen J et al (2009) Whole-body distribution and metabolism of [N-methyl-11C](R)-1-(2-chlorophenyl)-N-(1-methylpropyl)-3-isoquinolinecarboxamide in humans; an imaging agent for in vivo assessment of peripheral benzodiazepine receptor activity with positron emission tomography. Eur J Nucl Med Mol Imaging 36:671–682
Acknowledgments
We wish to thank our colleagues Tiina Ujula and Anu Autio for their excellent assistance in animal experiments. The study was conducted within the Finnish Center of Excellence in Molecular Imaging in Cardiovascular and Metabolic Research supported by the Academy of Finland, University of Turku, the Turku University Hospital, and the Åbo Akademi University. In addition, the study was financially supported by grants from the Academy of Finland (Nos. 205757 and 103032). Iina Laitinen is a PhD student supported by the National Drug Discovery Graduate School.
Author information
Authors and Affiliations
Corresponding author
Additional information
Significance: although carbon-11-labeled N-butan-2-yl-1-(2-chlorophenyl)-N-methylisoquinoline-3-carboxamide ([11C]PK11195) is a widely used PET tracer for inflammation imaging in humans, the associated radiation load remains unknown. This is the first study to provide estimates of human radiation dosimetry.
A part of the paper has been presented in the EANM Annual Congress 2005 in Istanbul, Turkey, and the corresponding abstract was published in Eur J Nucl Med Mol Imaging 2005;32:P752.
Rights and permissions
About this article
Cite this article
Luoto, P., Laitinen, I., Suilamo, S. et al. Human Dosimetry of Carbon-11 Labeled N-butan-2-yl-1-(2-chlorophenyl)-N-methylisoquinoline-3-carboxamide Extrapolated from Whole-body Distribution Kinetics and Radiometabolism in Rats. Mol Imaging Biol 12, 435–442 (2010). https://doi.org/10.1007/s11307-009-0293-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-009-0293-1