Abstract
Purpose
The purpose of the study is to synthesize and characterize near-infrared (NIR) fluorescence imaging probes targeted to gelatinases.
Procedures
A phage display-selected cyclic peptide containing the His-Try-Gly-Phe (HWGF) motif was used as the lead compound. Structure–activity relationship analysis was used to identify stable and potent gelatinase inhibitors suitable for NIR imaging applications.
Results
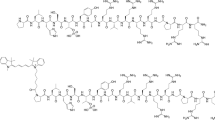
Replacing the S–S bond in cyclic peptide c(CTTHWGFTLC)NH2 (C1) with an amide bond between the ε-amino group of Lys and the side chain of Asp resulted in a significant increase in stability and a fourfold increase in gelatinase inhibition of the resulting peptide, c(KAHWGFTLD)NH2 (C6). Conjugation of Cy5.5 to C6 led to Cy5.5–C6, which was selectively taken up by MMP-2 expressing human glioma U87 cells. In vivo, selective accumulation of Cy5.5–C6, but not Cy5.5–C1 or a Cy5.5-scrambled peptide conjugate, was visualized in intratibial prostate PC-3 tumors 48 h after their intravenous injection. Moreover, Cy5.5–C6 was readily visualized in orthotopically inoculated U87 brain tumors.
Conclusions
Cy5.5–C6 may be a useful agent for molecular imaging of gelatinases. The approach of producing stable cyclic peptides through side chain amide linkage should be applicable to other peptide-based imaging agents.







Similar content being viewed by others
References
Ntziachristos V, Bremer C, Weissleder R (2003) Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. Eur Radiol 13:195–208
Sokolov K, Follen M, Aaron J et al (2003) Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res 63:1999–2004
Gurfinkel M, Ke S, Wen X et al (2003) Near-infrared fluorescence optical imaging and tomography. Dis Markers 19:107–121
Herschman HR (2004) Molecular imaging: a view from the inside. J Nucl Cardiol 11:210–214
Folli S, Westermann P, Braichotte D et al (1994) Antibody-indocyanin conjugates for immunophotodetection of human squamous cell carcinoma in nude mice. Cancer Res 54:2643–2649
Ballou B, Fisher GW, Waggoner AS et al (1995) Tumor labeling in vivo using cyanine-conjugated monoclonal antibodies. Cancer Immunol Immunother 41:257–263
Becker A, Hessenius C, Bhargava S et al (2000) Cyanine dye labeled vasoactive intestinal peptide and somatostatin analog for optical detection of gastroenteropancreatic tumors. Ann N Y Acad Sci 921:275–278
Achilefu S, Dorshow RB, Bugaj JE et al (2000) Novel receptor-targeted fluorescent contrast agents for in vivo tumor imaging. Invest Radiol 35:479–485
Zaheer A, Lenkinski RE, Mahmood A et al (2001) In vivo near-infrared fluorescence imaging of osteoblastic activity. Nat Biotechnol 19:1148–1154
Ke S, Wen X, Gurfinkel M et al (2003) Near-infrared optical imaging of epidermal growth factor receptor in breast cancer xenografts. Cancer Res 63:7870–7875
Weissleder R, Tung CH, Mahmood U et al (1999) In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol 17:375–378
Chen X, Conti PS, Moats RA (2004) In vivo near-infrared fluorescence imaging of integrin alphavbeta3 in brain tumor xenografts. Cancer Res 64:8009–8014
Wang W, Ke S, Wu Q et al (2004) Near-infrared optical imaging of integrin alphavbeta3 in human tumor xenografts. Mol Imaging 3:343–351
Murray GI, Duncan ME, O’Neil P et al (1996) Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med 2:461–462
Stetler-Stevenson WG, Aznavoorian S, Liotta LA (1993) Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 9:541–573
Karakiulakis G, Papanikolaou C, Jankovic SM et al (1997) Increased type IV collagen-degrading activity in metastases originating from primary tumors of the human colon. Invasion Metastasis 17:158–168
Brooks PC, Stromblad S, Sanders LC et al (1996) Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell 85:683–693
Vu TH, Shipley JM, Bergers G et al (1998) MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93:411–422
Koivunen E, Arap W, Valtanen H et al (1999) Tumor targeting with a selective gelatinase inhibitor. Nat Biotechnol 17:768–774
Oltenfreiter R, Staelens L, Kersemans V et al (2006) Valine-based biphenylsulphonamide matrix metalloproteinase inhibitors as tumor imaging agents. Appl Radiat Isot 64:677–685
Schafers M, Riemann B, Kopka K et al (2004) Scintigraphic imaging of matrix metalloproteinase activity in the arterial wall in vivo. Circulation 109:2554–2559
Bremer C, Tung CH, Weissleder R (2001) In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat Med 7:743–748
Bremer C, Bredow S, Mahmood U et al (2001) Optical imaging of matrix metalloproteinase-2 activity in tumors: feasibility study in a mouse model. Radiology 221:523–529
McIntyre JO, Matrisian LM (2003) Molecular imaging of proteolytic activity in cancer. J Cell Biochem 90:1087–1097
Kuhnast B, Bodenstein C, Haubner R et al (2004) Targeting of gelatinase activity with a radiolabeled cyclic HWGF peptide. Nucl Med Biol 31:337–344
Pirila E, Maisi P, Salo T et al (2001) In vivo localization of gelatinases (MMP-2 and -9) by in situ zymography with a selective gelatinase inhibitor. Biochem Biophys Res Commun 287:766–774
Hidalgo M, Eckhardt SG (2001) Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst 93:178–193
Mook ORF, Van Overbeek C, Ackema EG et al (2003) In situ localization of gelatinolytic activity in the extracellular matrix of metastases of colon cancer in rat liver using quenched fluorogenic DQ-gelatin. J Histochem Cytochem 51:821–829
Puyraimond A, Fridman R, Lemesle M et al (2001) MMP-2 colocalizes with caveolae on the surface of endothelial cells. Exp Cell Res 262:28–36
Upadhyay J, Shekarriz B, Nemeth JA et al (1999) Membrane type 1-matrix metalloproteinase (MT1-MMP) and MMP-2 immunolocalization in human prostate: change in cellular localization associated with high-grade prostatic intraepithelial neoplasia. Clin Cancer Res 5:4105–4110
Acknowledgement
Supported in part by NCI grants R01 EB000174 and U54 CA90810 and by the John S. Dunn Foundation. MALDI mass spectrometry was done by the Proteomics Core Laboratory in the Department of Molecular Pathology at M. D. Anderson Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, W., Shao, R., Wu, Q. et al. Targeting Gelatinases with a Near-Infrared Fluorescent Cyclic His-Try-Gly-Phe Peptide. Mol Imaging Biol 11, 424–433 (2009). https://doi.org/10.1007/s11307-009-0219-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-009-0219-y