Abstract
Introduction
The use of urea as a nitrogen (N) source by Chlorophytes usually enhances biomass and lipid production when compared to ammonium (NH4+). However, the metabolic shifts displayed by Chlamydomonas reinhardtii growing with this organic N source are not known.
Objectives
This study aimed: (i) to characterize the metabolism of C. reinhardtii cultivated in media containing only urea as N source as well as combined with different NH4+ ratios; (ii) to understand how metabolism respond to urea availability.
Methods
Specific quantification of metabolites using 96-well microplates, and high-performance liquid chromatography combined with non-targeted metabolite profiling by gas chromatography (GC)–time-of-flight (TOF)-mass spectrometry (MS) were used in this study. In addition, GC analysis was used to determine fatty acid profiling.
Results
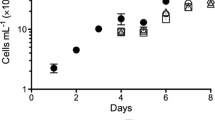
The use of urea did not alter the growth rate in comparison with NH4+. Interestingly, the cell number decreased and the cell size increased proportionally with urea availability. Furthermore, chlorophyll, protein and lipid contents increased with the amount of urea. Regarding the fatty acid profile, oleic acid (C18:1 w8) decreased with amount of urea, while linoleic acid (C18:2 w6) doubled in urea-containing medium.
Conclusions
These results indicate that urea promotes remarkable adjustments in metabolism, without drastic changes in biomass, promoting changes in carbohydrate and amino acid metabolism, as well as in lipids production and fatty acid profile.





Similar content being viewed by others
References
Atteia, A., Van Lis, R., Tielens, A. G. M., & Martin, W. F. (2013). Anaerobic energy metabolism in unicellular photosynthetic eukaryotes. Biochimica et Biophysica Acta, 1827, 210–223.
Barros, A., Guerra, T. L., Simões, M., Santos, E., Fonseca, D., Silva, J., Costa, L., & Navalho, J. (2017). Mass balance analysis of carbon and nitrogen in industrial scale mixotrophic microalgae cultures. Algal Research, 21, 35–41.
Berman, T., & Bronk, D. (2003). Dissolved organic nitrogen: A dynamic participant in aquatic ecosystems. Aquatic Microbial Ecology, 31, 279–305.
Blaby, I. K., Glaesener, A. G., Mettler, T., Fitz-Gibbon, S. T., Gallaher, S. D., Liu, B., Boyle, N. R., Kropat, J., Stitt, M., Johnson, S., Benning, C., Pellegrini, M., Casero, D., & Merchant, S. S. (2013). Systems-level analysis of nitrogen starvation-induced modifications of carbon metabolism in a Chlamydomonas reinhardtii starchless mutant. The Plant Cell, 25, 4305–4323.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Cagliari, A., Margis, R., Santos, D., Maraschin, F., Turchetto-Zolet, A. C., Loss, G., & Margis-Pinheiro, M. (2011). Biosynthesis of triacylglycerols (TAGs) in plants and algae. International Journal of Plant Biology, 2, 10.
Chen, W., Zhang, C., Song, L., Sommerfeld, M., & Hu, Q. (2009). A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. Journal of Microbiological Methods, 77, 41–47.
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25, 294–306.
Cross, J. M., von Korff, M., Altmann, T., Bartzetko, L., Sulpice, R., Gibon, Y., Palacios, N., & Stitt, M. (2006). Variation of enzyme activities and metabolite levels in 24 Arabidopsis accessions growing in carbon-limited conditions. Plant Physiology, 142, 1574–1588.
Cuadros-Inostroza, A., Caldana, C., Redestig, H., Kusano, M., Lisec, J., Peña-Cortés, H., Willmitzer, L., & Hannah, M. A. (2009). TargetSearch—A Bioconductor package for the efficient preprocessing of GC-MS metabolite profiling data. BMC Bioinformatics, 10, 428.
Dean, A. P., Sigee, D. C., Estrada, B., & Pittman, J. K. (2010). Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresource Technology, 101, 4499–4507.
Dhup, S., Kannan, D. C., & Dhawan, V. (2016). Understanding urea assimilation and its effect on lipid production and fatty acid composition of Scenedesmus sp. Symbiosis Journal of Biochemistry. https://doi.org/10.15226/2376-4589/2/1/00108.
Eustance, E., Gardner, R. D., Moll, K. M., Menicucci, J., Gerlach, R., & Peyton, B. M. (2013). Growth, nitrogen utilization and biodiesel potential for two chlorophytes grown on ammonium, nitrate or urea. Journal of Applied Phycology, 25, 1663–1677.
Fan, J., Cui, Y., Wan, M., Wang, W., & Li, Y. (2014). Lipid accumulation and biosynthesis genes response of the oleaginous Chlorella pyrenoidosa under three nutrition stressors. Biotechnology for Biofuels, 7, 17.
Fan, J., Yan, C., Andre, C., Shanklin, J., Schwender, J., & Xu, C. (2012). Oil accumulation is controlled by carbon precursor supply for fatty acid synthesis in Chlamydomonas reinhardtii. Plant and Cell Physiology, 53, 1380–1390.
Fernie, A. R., Roscher, A., Ratcliffe, R. G., & Kruger, N. J. (2001). Fructose 2,6-bisphosphate activates pyrophosphate: fructose-6-phosphate 1-phosphotransferase and increases triose phosphate to hexose phosphate cycling in heterotrophic cells. Planta, 212, 250–263.
Ferreira, D. F. (2014). Sisvar: A guide for its bootstrap procedures in multiple comparisons. Ciência e Agrotecnologia, 38, 109–112.
Florencio, F. J., & Vega, J. M. (1983). Utilization of nitrate, nitrite and ammonium by Chlamydomonas reinhardii: Photoproduction of ammonium. Planta, 158, 288–293.
Gérin, S., Mathy, G., Blomme, A., Franck, F., & Sluse, F. E. (2010). Plasticity of the mitoproteome to nitrogen sources (nitrate and ammonium) in Chlamydomonas reinhardtii: The logic of AOX1 gene localization. Biochimica et Biophysica Acta, 1797, 994–1003.
Gonçalves, E. C., Johnson, J. V., & Rathinasabapathi, B. (2013). Conversion of membrane lipid acyl groups to triacylglycerol and formation of lipid bodies upon nitrogen starvation in biofuel green algae Chlorella UTEX29. Planta, 238, 895–906.
Gonçalves, E. C., Koh, J., Zhu, N., Yoo, M. J., Chen, S., Matsuo, T., Johnson, J. V., & Rathinasabapathi, B. (2016). Nitrogen starvation-induced accumulation of triacylglycerol in the green algae: Evidence for a role for ROC40, a transcription factor involved in circadian rhythm. The Plant Journal, 85, 743–757.
Goodenough, U., Blaby, I., Casero, D., Gallaher, S. D., Goodson, C., Johnson, S., Lee, J. H., Merchant, S. S., Pellegrini, M., Roth, R., Rusch, J., Singh, M., Umen, G., Weiss, T. L., & Wulan, T. (2014). The path to triacylglyceride obesity in the sta6 strain of Chlamydomonas reinhardtii. Eukaryotic Cell, 13, 591–613.
Goodson, C., Roth, R., Wang, Z. T., & Goodenough, U. (2011). Structural correlates of cytoplasmic and chloroplast lipid body synthesis in Chlamydomonas reinhardtii and stimulation of lipid body production with acetate boost. Eukaryotic Cell, 10, 1592–1606.
Griffiths, M. J., Garcin, C., Van Hille, R. P., & Harrison, S. T. L. (2011). Interference by pigment in the estimation of microalgal biomass concentration by optical density. Journal of Microbiological Methods, 85, 119–123.
Harris, E. (1989). The Chlamydomonas sourcebook. A comprehensive guide to biology and laboratory (p. 780) San Diego: Academic Press.
Hodson, R. C., Williams, S. K., & Davidson, W. R. (1975). Metabolic control of urea catabolism in Chlamydomonas reinhardtii and Chlorella pyrenoidosa. Journal of Bacteriology, 121, 1022–1035.
Hsieh, C. H., & Wu, W. T. (2009). Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresource Technology, 100, 3921–3926.
Hu, Q., Sommerfeld, M., Jarvis, E., Ghirardi, M., Posewitz, M., Seibert, M., & Darzins, A. (2008). Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. The Plant Journal, 54, 621–639.
James, G. O., Hocart, C. H., Hillier, W., Chen, H., Kordbacheh, F., Price, G. D., & Djordjevic, M. A. (2011). Fatty acid profiling of Chlamydomonas reinhardtii under nitrogen deprivation. Bioresource Technology, 102, 3343–3351.
Juergens, M. T., Deshpande, R. R., Lucker, B. F., Park, J. J., Wang, H., Gargouri, M., Holguin, F. O., Disbrow, B., Schaub, T., Skepper, J. N., Kramer, D. M., Gang, D. R., Hicks, L. M., & Shachar-Hill, Y. (2015). The regulation of photosynthetic structure and function during nitrogen deprivation in Chlamydomonas reinhardtii. Plant Physiology, 167, 558–573.
Jung, H. (2002). The sodium/substrate symporter family: Structural and functional features. FEBS Letters, 529, 73–77.
Kamalanathan, M., Pierangelini, M., Shearman, L. A., et al. (2016). Impacts of nitrogen and phosphorus starvation on the physiology of Chlamydomonas reinhardtii. Journal of Applied Phycology, 28(3), 1509–1520.
Kirk, D. L., & Kirk, M. M. (1978). Carrier-mediated uptake of arginine and urea by Chlamydomonas reinhardtii. Plant Physiology, 61, 556–560.
Kjeldahl, J. (1883). “Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern” (New method for the determination of nitrogen in organic substances). Fresenius’ Zeitschrift für Analytische Chemie, 22, 366–383.
Kropat, J., Hong-Hermesdorf, A., Casero, D., Ent, P., Castruita, M., Pellegrini, M., Merchant, S. S., & Malasarn, D. (2011). A revised mineral nutrient supplement increases biomass and growth rate in Chlamydomonas reinhardtii. The Plant Journal, 66, 770–780.
Lari, Z., Moradi-Kheibari, N., Ahmadzadeh, H., Abrishamchi, P., Moheimani, N. R., Murry, M. A. (2016). Bioprocess engineering of microalgae to optimize lipid production through nutrient management. Journal of Applied Phycology, 28, 3235–3250.
Lee, D. Y., Park, J.-J., Barupal, D. K., & Fiehn, O. (2012). System response of metabolic networks in Chlamydomonas reinhardtii to total available ammonium. Molecular & Cellular Proteomics, 11, 973–988.
Li, W., Fingrut, D. R., & Maxwell, D. P. (2009). Characterization of a mutant of Chlamydomonas reinhardtii deficient in the molybdenum cofactor. Physiologia Plantarum, 136, 336–350.
Li, Y., Han, D., Hu, G., Dauvillee, D., Sommerfeld, M., Ball, S., & Hu, Q. (2010). Chlamydomonas starchless mutant defective in ADP-glucose pyrophosphorylase hyper-accumulates triacylglycerol. Metabolic Engineering, 12, 387–391.
Lisec, J., Schauer, N., Kopka, J., Willmitzer, L., & Fernie, A. R. (2006). Gas chromatography mass spectrometry-based metabolite profiling in plants. Nature Protocols, 1, 387–396.
Loera-Quezada, M. M., Angeles, G., & Olguín, E. J. (2011). Effect of irradiance on the cell density, size and lipid accumulation of Neochloris oleoabundans. Revista Latinoamericana de Biotecnología Ambiental y Algal, 2, 81–92.
Machado, M., Bromke, M., Domingues Júnior, A. P., Vaz, M. G. M. V., Rosa, R. M., Vinson, C. C., Sabir, J. S., Rocha, D. I., Martins, M. A., Araújo, W. L., Willmitzer, L., Szymanski, J., & Nunes-Nesi, A. (2016). Comprehensive metabolic reprograming in freshwater Nitzschia palea strains undergoing nitrogen starvation is likely associated with its ecological origin. Algal Research, 18, 116–126.
Markou, G., Vandamme, D., & Muylaert, K. (2014). Microalgal and cyanobacterial cultivation: The supply of nutrients. Water Research, 65, 186–202.
Masuko, T., Minami, A., Iwasaki, N., Majima, T., Nishimura, S. I., & Lee, Y. C. (2005). Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Analytical Biochemistry, 339, 69–72.
Matthew, T., Zhou, W., Rupprecht, J., Lim, L., Thomas-Hall, S. R., Doebbe, A., Kruse, O., Hankamer, B., Marx, U. C., Smith, S. M., & Schenk, P. M. (2009). The metabolome of Chlamydomonas reinhardtii following Induction of anaerobic H2 production by sulfur depletion. Journal of Biological Chemistry, 284, 23415–23425.
Merchán, F., Van den Ende, H., Fernández, E., & Beck, C. F. (2001). Low-expression genes induced by nitrogen starvation and subsequent sexual differentiation in Chlamydomonas reinhardtii, isolated by the differential display technique. Planta, 213, 309–317.
Merchant, S. S., Kropat, J., Liu, B., Shaw, J., & Warakanont, J. (2012). TAG, You’re it! Chlamydomonas as a reference organism for understanding algal triacylglycerol accumulation. Current Opinion in Biotechnology, 23, 352–363.
Metting, F. B. (1996). Biodiversity and application of microalgae. Journal of Industrial Microbiology and Biotechnology, 17, 477–489.
Michielsen, M. J. F., Meijer, E. A., Wijffels, R. H., Tramper, J., & Beeftink, H. H. (1998). Kinetics of d-malate production by permeabilized Pseudomonas pseudoalcaligenes. Enzyme and Microbial Technology, 22, 621–628.
Miller, R., Wu, G., Deshpande, R. R., Vieler, A., Gärtner, K., Li, X., Moellering, E. R., Zäuner, S., Cornish, A. J., Liu, B., Bullard, B., Sears, B. B., Kuo, M. H., Hegg, E. L., Shachar-Hill, Y., Shiu, S. H., & Benning, C. (2010). Changes in transcript abundance in Chlamydomonas reinhardtii following nitrogen deprivation predict diversion of metabolism. Plant Physiology, 154, 1737–1752.
Min, S. K., Yoon, G. H., Joo, J. H., Sim, S. J., & Shin, H. S. (2014). Mechanosensitive physiology of Chlamydomonas reinhardtii under direct membrane distortion. Scientific Reports, 4, 4675.
Moellering, E. R., & Benning, C. (2010). RNA interference silencing of a major lipid droplet protein affects lipid droplet size in Chlamydomonas reinhardtii. Eukaryotic Cell, 9, 97–106.
Molloy, C. J., & Syrett, P. J. (1988). Interrelationships between uptake of urea and uptake of ammonium by microalgae. Journal of Experimental Marine Biology and Ecology, 118, 85–95.
Moon, M., Kim, C. W., Park, W. K., Yoo, G., Choi, Y., & Yang, J. W. (2013). Mixotrophic growth with acetate or volatile fatty acids maximizes growth and lipid production in Chlamydomonas reinhardtii. Algal Research, 2, 352–357.
Moroney, J. V., & Ynalvez, R. A. (2007). Proposed carbon dioxide concentrating mechanism in Chlamudomonas reinhardtii. Eukaryotic Cell, 6, 1251–1259.
Nigam, S., Prakash Rai, M., & Sharma, R. (2011). Effect of nitrogen on growth and lipid content of Chlorella pyrenoidosa. American Journal of Biochemistry and Biotechnology, 7, 126–131.
Park, J. J., Wang, H., Gargouri, M., Deshpande, R. R., Skepper, J. N., Holguin, F. O., Juergens, M. T., Shachar-Hill, Y., Hicks, L. M., & Gang, D. R. (2015). The response of Chlamydomonas reinhardtii to nitrogen deprivation: A systems biology analysis. The Plant Journal, 81, 611–624.
Perrine, Z., Negi, S., & Sayre, R. T. (2012). Optimization of photosynthetic light energy utilization by microalgae. Algal Research, 1(2), 134–142.
Philipps, G., Happe, T., & Hemschemeier, A. (2012). Nitrogen deprivation results in photosynthetic hydrogen production in Chlamydomonas reinhardtii. Planta, 235, 729–745.
Porra, R. J., Thompson, W., & Kriedemann, P. E. (1989). Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll-a and chlorophyll-b extracted with 4 different solvents—Verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy. Biochimica et Biophysica Acta, 975, 384–394.
Recht, L., Töpfer, N., Batushansky, A., Sikron, N., Gibon, Y., Fait, A., Nikoloski, Z., Boussiba, S., & Zarka, A. (2014). Metabolite profiling and integrative modeling reveal metabolic constraints for carbon partitioning under nitrogen starvation in the green algae Haematococcus pluvialis. Journal of Biological Chemistry, 289, 30387–30403.
Remacle, C., Eppe, G., Coosemans, N., Fernandez, E., Vigeolas, H. (2014) Combined intracellular nitrate and NIT2 effects on storage carbohydrate metabolism in Chlamydomonas. Journal of Experimental Botany, 65(1), 23–33.
Rocha, R. P., Machado, M., Vaz, M. G. M. V., Vinson, C. C., Leite, M., Richard, R., Mendes, L. B. B., Araújo, W. L., Caldana, C., Martins, M. A., Williams, T. C. R., & Nunes-Nesi, A. (2017). Exploring the metabolic and physiological diversity of native microalgal strains (Chlorophyta) isolated from tropical freshwater reservoirs. Algal Research, 28, 139–150.
Salas-Montantes, C. J., González-Ortega, O., Ochoa-Alfaro, A. E., et al. (2018). Lipid accumulation during nitrogen and sulfur starvation in Chlamydomonas reinhardtii overexpressing a transcription factor. Journal of Applied Phycology, 30, 1721.
Sanz-Luque, E., Chamizo-Ampudia, A., Llamas, A., Galvan, A., & Fernandez, E. (2015). Understanding nitrate assimilation and its regulation in microalgae. Frontiers in Plant Science, 6, 899.
Schmollinger, S., Mühlhaus, T., Boyle, N. R., Casero, D., Mettler, T., Moseley, J. L., Kropat, J., Sommer, F., Strenkert, D., Hemme, D., Pellegrini, M., Grossman, A. R., Stitt, M., Schroda, M., & Merchant, S. S. (2014). Nitrogen-sparing mechanisms in Chlamydomonas affect the transcriptome, the proteome, and photosynthetic metabolism. The Plant Cell, 26, 1410–1435.
Scragg, A., Illman, A., Carden, A., & Shales, S. (2002). Growth of microalgae with increased calorific values in a tubular bioreactor. Biomass and Bioenergy, 23, 67–73.
Siaut, M., Cuiné, S., Cagnon, C., Fessler, B., Nguyen, M., Carrier, P., Beyly, A., Beisson, F., Triantaphylidès, C., Li-Beisson, Y., & Peltier, G. (2011). Oil accumulation in the model green alga Chlamydomonas reinhardtii: Characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnology, 11, 7.
Siegfried, B. R., Ruckemann, H., & Stumpf, G. (1984). Method for the determination of organic acids in silage by high performance liquid chromatography. Landwirtschaftliche Forschung, 37, 298.
Solomon, C. M., Collier, J. L., Berg, G. M., & Glibert, P. M. (2010). Role of urea in microbial metabolism in aquatic systems: A biochemical and molecular review. Aquatic Microbial Ecology, 59, 67–88.
Song, P., Li, L., & Liu, J. (2013). Proteomic analysis in nitrogen-deprived Isochrysis galbana during lipid accumulation. PLoS ONE, 8, 82188.
Spolaore, P., Joannis-Cassan, C., Duran, E., & Isambert, A. (2006). Commercial applications of microalgae. Journal of Bioscience and Bioengineering, 101, 87–96.
Stacklies, W., Redestig, H., Scholz, M., Walther, D., & Selbig, J. (2007). PCA methods—A bioconductor package providing PCA methods for incomplete data. Bioinformatics, 23, 1164–1167.
Strope, P. K., Nickerson, K. W., Harris, S. D., & Moriyama, E. N. (2011). Molecular evolution of urea amidolyase and urea carboxylase in fungi. BMC Evolutionary Biology, 11, 80.
Taylor, M. W., Barr, N. G., Grant, C. M., & Rees, T. A. V. (2006). Changes in amino acid composition of Ulva intestinalis (Chlorophyceae) following addition of ammonium or nitrate. Phycologia, 45, 270–276.
Teoh, M. L., Chu, W. L., Marchant, H., & Phang, S. M. (2004). Influence of culture temperature on the growth, biochemical composition and fatty acid profiles of six Antarctic microalgae. Journal of Applied Phycology, 16, 421–430.
Tovar-Méndez, A., Miernyk, J. A., & Randall, D. D. (2003). Regulation of pyruvate dehydrogenase complex activity in plant cells. European Journal of Biochemistry, 270, 1043–1049.
Urzica, E. I., Vieler, A., Hong-Hermesdorf, A., Page, M. D., Casero, D., Gallaher, S. D., Kropat, J., Pellegrini, M., Benning, C., & Merchant, S. S. (2013). Remodeling of membrane lipids in iron-starved Chlamydomonas. Journal of Biological Chemistry, 288, 30246–30258.
Wang, W. H., Köhler, B., Cao, F. Q., & Liu, L. H. (2008). Molecular and physiological aspects of urea transport in higher plants. Plant Science, 175, 467–477.
Wang, Z. T., Ullrich, N., Joo, S., Waffenschmidt, S., & Goodenough, U. (2009). Algal lipid bodies: Stress induction, purification, and biochemical characterization in wild-type and starchless Chlamydomonas reinhardtii. Eukaryotic Cell, 8, 1856–1868.
Wase, N., Black, P. N., Stanley, B. A., & Dirusso, C. C. (2014). Integrated quantitative analysis of nitrogen stress response in Chlamydomonas reinhardtii using metabolite and protein profiling. Journal of Proteome Research, 13, 1373–1396.
Wei, L., Derrien, B., Gautier, A., Houille-Vernes, L., Boulouis, A., Saint-Marcoux, D., Malnoë, A., Rappaport, F., de Vitry, C., Vallon, O., Choquet, Y., & Wollman, F. A. (2014). Nitric oxide-triggered remodeling of chloroplast bioenergetics and thylakoid proteins upon nitrogen starvation in Chlamydomonas reinhardtii. The Plant Cell, 26, 353–372.
Winck, F. V., Melo, D. O. P., & Barrios, A. F. G. (2013). Carbon acquisition and accumulation in microalgae Chlamydomonas: Insights from “omics” approaches. Journal of Proteomics, 94, 207–218.
Work, V. H., Radakovits, R., Jinkerson, R. E., Meuser, J. E., Elliott, L. G., Vinyard, D. J., Laurens, L. M. L., Dismuker, G. C., & Posewitz, M. C. (2010). Increased lipid accumulation in the Chlamydomonas reinhardtii sta7-10 starchless isoamylase mutant and increased carbohydrate synthesis in complemented strains. Eukaryotic Cell, 9, 1251–1261.
Worley, B., & Powers, R. (2013). Multivariate analysis in metabolomics. Current Metabolomics, 1, 92–107.
Xiao, D. D., Xiao, W. F., & Ya, J. L. (2011). The effects of nutritional restriction on neutral lipid accumulation in Chlamydomonas and Chlorella. African Journal of Microbiology Research, 5, 260–270.
Xu, H., Miao, X., & Wu, Q. (2006). High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. Journal of Biotechnology, 126, 499–507.
Zhan, J., Hong, Y., & Hu, H. (2016). Effects of nitrogen sources and C/N ratios on the lipid-producing potential of Chlorella sp. Journal of Microbiology and Biotechnology, 26, 1290–1302.
Zhang, X. W., Chen, F., & Johns, M. R. (1999). Kinetic models for heterotrophic growth of Chlamydomonas reinhardtii in batch and fed-batch cultures. Process Biochemistry, 35, 385–389.
Acknowledgements
This work was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) [Grant APQ-01357-14, APQ-01671-15 and RED-00053-16] and Max Planck Society to WLA. Research fellowships granted by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to ANN, WLA, MM, and ASM and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) to ADB and RMR are also gratefully acknowledged. M.G.M.V.V. was supported by scholarships from CAPES/Fundação de Amparo à Pesquisa do estado de Minas Gerais (FAPEMIG) (BPD-00514-14) and CAPES (PNPD-1638006). The authors wish to thank the NUBIOMOL-UFV for providing the facilities for the analysis of this work.
Author information
Authors and Affiliations
Contributions
ADB and ANN conceived and designed research. ADB, RMR, ASM, BAS and PFG conducted experiments. ADB, MM and MGMVV analyzed data. ADB and ANN wrote the manuscript. LC and WLA revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Research involving human and animal participants
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Batista, A.D., Rosa, R.M., Machado, M. et al. Increased urea availability promotes adjustments in C/N metabolism and lipid content without impacting growth in Chlamydomonas reinhardtii. Metabolomics 15, 31 (2019). https://doi.org/10.1007/s11306-019-1496-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-019-1496-3