Abstract
Introduction
Methanol utilization by bacteria is important for various industrial processes. Methylotrophic bacteria are taxonomically diverse and some species promote plant growth and induce stress tolerance. However, methylotrophic potential of bacterial endophytes is poorly understood.
Objective
The current study aimed to evaluate the metabolomic and proteomic changes in endophytic Bacillus amyloliquefaciens RWL-1 caused by its methanol utilization and the resultant influence on its phytohormone production.
Methods
B. amyloliquefaciens RWL-1 was grown in LB medium with different concentrations [0 (control), 0.5, 1, 1.5, 2, 2.5, 3, 3.5, and 4%) of methanol to examine its methylotrophic potential. SDS-PAGE analysis was carried out for bacterial protein confirmation. Moreover, the phytohormones (indole 3 acetic acid (IAA), gibberellins (GAs), abscisic acid (ABA)) produced by RWL-1 in methanol supplemented medium were quantified by GC-MS/SIM (6890N Network GC system, and 5973 Network Mass Selective Detector; Agilent Technologies, Santa Clara, CA, USA), while the antioxidants were estimated spectrophotometrically (T60 UV-VIS spectrophotometer, Leicester, UK). The amino acid quantification was carried out by amino acid analyzer (HITACHI L-8900, Japan). Furthermore, Nano-liquid chromatography (LC)–MS/MS analysis was performed with an Agilent system (Wilmington, DE, USA) for proteomic analysis while mascot algorithm (Matrix science, USA) was used to identify peptide sequences present in the protein sequence database.
Results
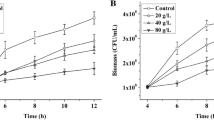
RWL-1 showed significant growth in media supplemented with 2 and 3.5% methanol, when compared with other concentrations. Mass spectroscopy analysis revealed that RWL-1 utilizes methanol efficiently as a carbon source. In the presence of methanol, RWL-1 produced significantly higher levels of IAA but lower levels of ABA, when compared with the control. Further, enzymatic antioxidants and functional amino acids were significantly up-regulated, with predominant expression of glutamic acid and alanine. Nano-liquid chromatography, quadrupole time-of-flight analysis, and quantitative analysis of methanol-treated bacterial cells showed expression of eight different types of proteins, including detoxification proteins, unrecognized and unclassified enzymes with antioxidant properties, proteases, metabolism enzymes, ribosomal proteins, antioxidant proteins, chaperones, and heat shock proteins.
Conclusion
Results demonstrate that RWL-1 can significantly enhance its growth by utilizing methanol, and could produce phytohormones when growing in methanol-supplemented media, with increased expression of specific proteins and different biochemicals. These results will be useful in devising strategies for utilizing methylotrophic bacterial endophytes as alternative promoters of plant growth.
Graphical abstract
Understanding RWL-1 ability to utilize methanol. The survival and phytohormones production by Bacillus amyloliquefaciens RWL-1 in methanol supplemented media whistle inducing metabolic and proteomic changes.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this manuscript.
References
Abanda-Nkpwatt, D., Müsch, M., Tschiersch, J., Boettner, M., & Schwab, W. (2006). Molecular interaction between Methylobacterium extorquens and seedlings: Growth promotion, methanol consumption, and localization of the methanol emission site. Journal of Experimental Botany, 57(15), 4025–4032.
Aguilera, L., Ferreira, E., Giménez, R., Fernández, F. J., Taulés, M., Aguilar, J., et al. (2012). Secretion of the housekeeping protein glyceraldehyde-3-phosphate dehydrogenase by the LEE-encoded type III secretion system in enteropathogenic Escherichia coli. The International Journal of Biochemistry and Cell Biology, 44(6), 955–962.
Aguilera Gil, M. L., Giménez Claudio, R., Badía Palacín, J., Aguilera Piera, J., & Baldomà Llavinés, L. (2009). NAD+-dependent post-translational modification of Escherichia coli glyceraldehyde-3-phosphate dehydrogenase. International Microbiology, 2009, 129(3), 187–192.
Brader, G., Compant, S., Mitter, B., Trognitz, F., & Sessitsch, A. (2014). Metabolic potential of endophytic bacteria. Current Opinion in Biotechnology, 27, 30–37.
Bruins, M. R., Kapil, S., & Oehme, F. W. (2000). Microbial resistance to metals in the environment. Ecotoxicology and Environmental Safety, 45(3), 198–207.
Cabiscol Català, E., Tamarit Sumalla, J., & Ros Salvador, J. (2000). Oxidative stress in bacteria and protein damage by reactive oxygen species. International Microbiology, 2000, 3(1), 3–8.
Chen, L., Xie, Q., & Nathan, C. (1998). Alkyl hydroperoxide reductase subunit C (AhpC) protects bacterial and human cells against reactive nitrogen intermediates. Molecular Cell, 1(6), 795–805.
Chen, X. H., Koumoutsi, A., Scholz, R., Eisenreich, A., Schneider, K., Heinemeyer, I., et al. (2007). Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nature Biotechnology, 25(9), 1007–1014.
Chistoserdova, L., Chen, S.-W., Lapidus, A., & Lidstrom, M. E. (2003). Methylotrophy in Methylobacterium extorquens AM1 from a genomic point of view. Journal of Bacteriology, 185(10), 2980–2987.
Chistoserdova, L., Vorholt, J. A., Thauer, R. K., & Lidstrom, M. E. (1998). C1 transfer enzymes and coenzymes linking methylotrophic bacteria and methanogenic Archaea. Science, 281(5373), 99–102.
Choi, S.-K., Jeong, H., Kloepper, J. W., & Ryu, C.-M. (2014). Genome sequence of Bacillus amyloliquefaciens GB03, an active ingredient of the first commercial biological control product. Genome Announcements, 2(5), e01092–e01014.
Cohen, A. C., Bottini, R., & Piccoli, P. N. (2008). Azospirillum brasilense Sp 245 produces ABA in chemically-defined culture medium and increases ABA content in Arabidopsis plants. Plant Growth Regulation, 54(2), 97–103.
Dominy, J. E., Hwang, J., Guo, S., Hirschberger, L. L., Zhang, S., & Stipanuk, M. H. (2008). Synthesis of amino acid cofactor in cysteine dioxygenase is regulated by substrate and represents a novel post-translational regulation of activity. Journal of Biological Chemistry, 283(18), 12188–12201.
Dourado, M. N., Camargo Neves, A. A., Santos, D. S., & Araújo, W. L. (2015). Biotechnological and agronomic potential of endophytic pink-pigmented methylotrophic Methylobacterium spp. BioMed Research International. https://doi.org/10.1155/2015/909016.
Egea, L., Aguilera, L., Gimenez, R., Sorolla, M. A., Aguilar, J., Badia, J., & Baldoma, L. (2007). Role of secreted glyceraldehyde-3-phosphate dehydrogenase in the infection mechanism of enterohemorrhagic and enteropathogenic Escherichia coli: Interaction of the extracellular enzyme with human plasminogen and fibrinogen. The International Journal of Biochemistry and Cell Biology, 39(6), 1190–1203.
Eisenstein, E., Gilliland, G. L., Herzberg, O., Moult, J., Orban, J., Poljak, R. J., et al. (2000). Biological function made crystal clear—Annotation of hypothetical proteins via structural genomics. Current Opinion in Biotechnology, 11(1), 25–30.
Galbally, I. E., & Kirstine, W. (2002). The production of methanol by flowering plants and the global cycle of methanol. Journal of Atmospheric Chemistry, 43(3), 195–229.
Ghosh, K., Tyagi, N., Kumar, H., & Rathi, S. (2015). DNA interaction, SOD, peroxidase and nuclease activity studies of iron complex having ligand with carboxamido nitrogen donors. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 146, 292–296. https://doi.org/10.1016/j.saa.2015.03.003.
Gourion, B., Rossignol, M., & Vorholt, J. A. (2006). A proteomic study of Methylobacterium extorquens reveals a response regulator essential for epiphytic growth. Proceedings of the National Academy of Sciences of USA, 103(35), 13186–13191.
Griffin, T. J., Gygi, S. P., Ideker, T., Rist, B., Eng, J., Hood, L., & Aebersold, R. (2002). Complementary profiling of gene expression at the transcriptome and proteome levels in Saccharomyces cerevisiae. Molecular and Cellular Proteomics, 1(4), 323–333.
Guo, H., Luo, S., Chen, L., Xiao, X., Xi, Q., Wei, W., et al. (2010). Bioremediation of heavy metals by growing hyperaccumulaor endophytic bacterium Bacillus sp. L14. Bioresource Technology, 101(22), 8599–8605.
Gygi, S. P., Rist, B., Gerber, S. A., Turecek, F., Gelb, M. H., & Aebersold, R. (1999). Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nature Biotechnology, 17(10), 994.
Hall, J. L. (2002). Cellular mechanisms for heavy metal detoxification and tolerance. Journal of Experimental Botany, 53(366), 1–11.
Halo, B. A., Khan, A. L., Waqas, M., Al-Harrasi, A., Hussain, J., Ali, L., et al. (2015). Endophytic bacteria (Sphingomonas sp. LK11) and gibberellin can improve Solanum lycopersicum growth and oxidative stress under salinity. Journal of Plant Interactions, 10(1), 117–125.
Heggeset, T. M. B., Krog, A., Balzer, S., Wentzel, A., Ellingsen, T. E., & Brautaset, T. (2012). Genome sequence of thermotolerant Bacillus methanolicus: Features and regulation related to methylotrophy and production of l-lysine and l-glutamate from methanol. Applied and Environmental Microbiology. https://doi.org/10.1128/AEM.00703-12.
Hossain, M. J., Ran, C., Liu, K., Ryu, C.-M., Rasmussen-Ivey, C. R., Williams, M. A., et al. (2015). Deciphering the conserved genetic loci implicated in plant disease control through comparative genomics of Bacillus amyloliquefaciens subsp. plantarum. Frontiers in Plant Science, 6, 631.
Hovey, R., Lentes, S., Ehrenreich, A., Salmon, K., Saba, K., Gottschalk, G., et al. (2005). DNA microarray analysis of Methanosarcina mazei Gö1 reveals adaptation to different methanogenic substrates. Molecular Genetics and Genomics, 273(3), 225–239.
Hüve, K., Christ, M. M., Kleist, E., Uerlings, R., Niinemets, Ü, Walter, A., & Wildt, J. (2007). Simultaneous growth and emission measurements demonstrate an interactive control of methanol release by leaf expansion and stomata. Journal of Experimental Botany, 58(7), 1783–1793.
Idriss, E. E., Makarewicz, O., Farouk, A., Rosner, K., Greiner, R., Bochow, H., et al. (2002). Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant-growth-promoting effect. Microbiology, 148(7), 2097–2109.
Irla, M., Neshat, A., Brautaset, T., Rückert, C., Kalinowski, J., & Wendisch, V. F. (2015). Transcriptome analysis of thermophilic methylotrophic Bacillus methanolicus MGA3 using RNA-sequencing provides detailed insights into its previously uncharted transcriptional landscape. BMC Genomics, 16(1), 73.
Irla, M., Neshat, A., Winkler, A., Albersmeier, A., Heggeset, T. M. B., Brautaset, T., et al. (2014). Complete genome sequence of Bacillus methanolicus MGA3, a thermotolerant amino acid producing methylotroph. Journal of Biotechnology, 188, 110–111.
Ivanova, E. G., Doronina, N. V., & Trotsenko, Y. A. (2001). Aerobic methylobacteria are capable of synthesizing auxins. Microbiology, 70(4), 392–397.
Janahiraman, V., Anandham, R., Kwon, S. W., Sundaram, S., Karthik Pandi, V., Krishnamoorthy, R., et al. (2016). Control of wilt and rot pathogens of tomato by antagonistic pink pigmented facultative methylotrophic Delftia lacustris and Bacillus spp. Frontiers in Plant Science, 7, 1626.
Kelly, D. P., & Murrell, J. C. (1999). Microbial metabolism of methanesulfonic acid. Archives of Microbiology, 172(6), 341–348.
Khan, A. L., Hussain, J., Al-Harrasi, A., Al-Rawahi, A., & Lee, I.-J. (2015). Endophytic fungi: Resource for gibberellins and crop abiotic stress resistance. Critical Reviews in Biotechnology, 35(1), 62–74. https://doi.org/10.3109/07388551.2013.800018.
Khan, A. L., Ullah, I., Hussain, J., Kang, S., Al-Harrasi, A., Al-Rawahi, A., & Lee, I. (2016). Regulations of essential amino acids and proteomics of bacterial endophytes Sphingomonas sp. Lk11 during cadmium uptake. Environmental Toxicology, 31(7), 887–896.
Kim, A.-Y., Shahzad, R., Kang, S.-M., Khan, A. L., Lee, S.-M., Park, Y.-G., et al. (2017). Paenibacillus terrae AY-38 resistance against botrytis cinerea in Solanum lycopersicum L. plants through defence hormones regulation. Journal of Plant Interactions. https://doi.org/10.1080/17429145.2017.1319502.
Kinoshita, H., Uchida, H., Kawai, Y., Kawasaki, T., Wakahara, N., Matsuo, H., et al. (2008). Cell surface Lactobacillus plantarum LA 318 glyceraldehyde-3-phosphate dehydrogenase (GAPDH) adheres to human colonic mucin. Journal of Applied Microbiology, 104(6), 1667–1674.
Koenig, R. L., Morris, R. O., & Polacco, J. C. (2002). tRNA is the source of low-level trans-zeatin production in Methylobacterium spp. Journal of Bacteriology, 184(7), 1832–1842.
Koh, M., Kim, C. S., Kim, Y. A., Choi, H. S., Cho, E. H., Kim, E., et al. (2002). Properties of electron carriers in the process of methanol oxidation in a new restricted facultative marine methylotrophic bacterium, Methylophaga sp. MP. Journal of Microbiology and Biotechnology, 12(3), 476–482.
Kumar, M., Tomar, R. S., Lade, H., & Paul, D. (2016). Methylotrophic bacteria in sustainable agriculture. World Journal of Microbiology and Biotechnology, 32(7), 120.
Lee, H. S., Madhaiyan, M., Kim, C. W., Choi, S. J., Chung, K. Y., & Sa, T. M. (2006). Physiological enhancement of early growth of rice seedlings (Oryza sativa L.) by production of phytohormone of N2-fixing methylotrophic isolates. Biology and Fertility of Soils, 42(5), 402–408.
Li, L., Li, Q., Rohlin, L., Kim, U., Salmon, K., Rejtar, T., et al. (2007). Quantitative proteomic and microarray analysis of the Archaeon Methanosarcina acetivorans grown with acetate versus methanol. Journal of Proteome Research, 6(2), 759–771.
Long, H. H., Schmidt, D. D., & Baldwin, I. T. (2008). Native bacterial endophytes promote host growth in a species-specific manner; phytohormone manipulations do not result in common growth responses. PLoS ONE, 3(7), e2702.
Lugtenberg, B., & Mercado-Blanco, J. (2014). Biotechnological applications of bacterial endophytes. Current Biotechnology. https://doi.org/10.2174/22115501113026660038.
Madhaiyan, M., Poonguzhali, S., Kwon, S.-W., & Sa, T.-M. (2010). Bacillus methylotrophicus sp. nov., a methanol-utilizing, plant-growth-promoting bacterium isolated from rice rhizosphere soil. International Journal of Systematic and Evolutionary Microbiology, 60(10), 2490–2495.
Madhaiyan, M., Poonguzhali, S., Senthilkumar, M., Seshadri, S., Chung, H., Yang, J., et al. (2004). Growth promotion and induction of systemic resistance in rice cultivar Co-47 (Oryza sativa L.) by Methylobacterium spp. Botanical Bulletin of Academia Sinica, 45, 315–324.
Madhaiyan, M., Reddy, B. V. S., Anandham, R., Senthilkumar, M., Poonguzhali, S., Sundaram, S. P., & Sa, T. (2006). Plant growth-promoting Methylobacterium induces defense responses in groundnut (Arachis hypogaea L.) compared with rot pathogens. Current Microbiology, 53(4), 270–276.
Mano, H., & Morisaki, H. (2008). Endophytic bacteria in the rice plant. Microbes and Environments, 23(2), 109–117.
Maurizi, M. R. (1998). Biochemical properties and biological functions of ATP-dependent proteases in bacterial cells. In E. E. Bittar & A. J. Rivett (Eds.), Intracellular protein degradation (Vol. 27, pp. 1–41). Elsevier. https://doi.org/10.1016/S1569-2558(08)60456-7.
McDonald, I. R., & Murrell, J. C. (1997). The methanol dehydrogenase structural gene mxaF and its use as a functional gene probe for methanotrophs and methylotrophs. Applied and Environmental Microbiology, 63(8), 3218–3224. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC168619/.
McTaggart, T. L., Beck, D. A. C., Setboonsarng, U., Shapiro, N., Woyke, T., Lidstrom, M. E., et al. (2015). Genomics of methylotrophy in Gram-positive methylamine-utilizing bacteria. Microorganisms, 3(1), 94–112.
Meena, K. K., Kumar, M., Kalyuzhnaya, M. G., Yandigeri, M. S., Singh, D. P., Saxena, A. K., & Arora, D. K. (2012). Epiphytic pink-pigmented methylotrophic bacteria enhance germination and seedling growth of wheat (Triticum aestivum) by producing phytohormone. Antonie van Leeuwenhoek, 101(4), 777–786.
Müller, J. E. N., Litsanov, B., Bortfeld-Miller, M., Trachsel, C., Grossmann, J., Brautaset, T., & Vorholt, J. A. (2014). Proteomic analysis of the thermophilic methylotroph Bacillus methanolicus MGA 3. Proteomics, 14(6), 725–737.
Nemecek-Marshall, M., MacDonald, R. C., Franzen, J. J., Wojciechowski, C. L., & Fall, R. (1995). Methanol emission from leaves (enzymatic detection of gas-phase methanol and relation of methanol fluxes to stomatal conductance and leaf development). Plant Physiology, 108(4), 1359–1368.
Okumura, M., Fujitani, Y., Maekawa, M., Charoenpanich, J., Murage, H., Kimbara, K., et al. (2017). Cultivable Methylobacterium species diversity in rice seeds identified with whole-cell matrix-assisted laser desorption/ionization time-of-flight mass spectrometric analysis. Journal of Bioscience and Bioengineering, 123(2), 190–196.
Omer, Z. S., Tombolini, R., Broberg, A., & Gerhardson, B. (2004). Indole-3-acetic acid production by pink-pigmented facultative methylotrophic bacteria. Plant Growth Regulation, 43(1), 93–96.
Pancholi, V., & Chhatwal, G. S. (2003). Housekeeping enzymes as virulence factors for pathogens. International Journal of Medical Microbiology, 293(6), 391–401.
Pirttilä, A. M., Joensuu, P., Pospiech, H., Jalonen, J., & Hohtola, A. (2004). Bud endophytes of Scots pine produce adenine derivatives and other compounds that affect morphology and mitigate browning of callus cultures. Physiologia Plantarum, 121(2), 305–312.
Rajkumar, M., Ae, N., & Freitas, H. (2009). Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere, 77(2), 153–160.
Reva, O. N., Dixelius, C., Meijer, J., & Priest, F. G. (2004). Taxonomic characterization and plant colonizing abilities of some bacteria related to Bacillus amyloliquefaciens and Bacillus subtilis. FEMS Microbiology Ecology, 48(2), 249–259.
Sánchez, B., Schmitter, J., & Urdaci, M. C. (2009). Identification of novel proteins secreted by Lactobacillus rhamnosus GG grown in de Mann–Rogosa–Sharpe broth. Letters in Applied Microbiology, 48(5), 618–622.
Schauer, S., & Kutschera, U. (2011). A novel growth-promoting microbe, Methylobacterium funariae sp. nov., isolated from the leaf surface of a common moss. Plant Signaling and Behavior, 6(4), 510–515.
Schrader, J., Schilling, M., Holtmann, D., Sell, D., Villela Filho, M., Marx, A., & Vorholt, J. A. (2009). Methanol-based industrial biotechnology: Current status and future perspectives of methylotrophic bacteria. Trends in Biotechnology, 27(2), 107–115.
Seib, K. L., Wu, H.-J., Kidd, S. P., Apicella, M. A., Jennings, M. P., & McEwan, A. G. (2006). Defenses against oxidative stress in Neisseria gonorrhoeae: A system tailored for a challenging environment. Microbiology and Molecular Biology Reviews, 70(2), 344–361.
Shahzad, R., Khan, A. L., Bilal, S., Asaf, S., & Lee, I.-J. (2017a). Plant growth-promoting endophytic bacteria versus pathogenic infections: An example of Bacillus amyloliquefaciens RWL-1 and Fusarium oxysporum f. sp. lycopersici in tomato. PeerJ. https://doi.org/10.7717/peerj.3107.
Shahzad, R., Khan, A. L., Bilal, S., Waqas, M., Kang, S.-M., & Lee, I.-J. (2017b). Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environmental and Experimental Botany. https://doi.org/10.1016/j.envexpbot.2017.01.010.
Shahzad, R., Waqas, M., Khan, A. L., Asaf, S., Khan, M. A., Kang, S.-M., et al. (2016). Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiology and Biochemistry. https://doi.org/10.1016/j.plaphy.2016.05.006.
Skovran, E., Palmer, A. D., Rountree, A. M., Good, N. M., & Lidstrom, M. E. (2011). XoxF is required for expression of methanol dehydrogenase in Methylobacterium extorquens AM1. Journal of Bacteriology. https://doi.org/10.1128/JB.05367-11.
Šmejkalová, H., Erb, T. J., & Fuchs, G. (2010). Methanol assimilation in Methylobacterium extorquens AM1: Demonstration of all enzymes and their regulation. PLoS ONE, 5(10), e13001.
Srinivasan, B., Nagappa, L. K., Shukla, A., & Balaram, H. (2015). Prediction of substrate specificity and preliminary kinetic characterization of the hypothetical protein PVX_123945 from Plasmodium vivax. Experimental Parasitology, 151, 56–63.
Stafford, S. J., Humphreys, D. P., & Lund, P. A. (1999). Mutations in dsbA and dsbB, but not dsbC, lead to an enhanced sensitivity of Escherichia coli to Hg2+ and Cd2+. FEMS Microbiology Letters, 174(1), 179–184.
Subhaswaraj, P., Jobina, R., Parasuraman, P., & Siddhardha, B. (2017). Plant growth promoting activity of pink pigmented facultative methylotroph—Methylobacterium extorquens MM2 on Lycopersicon esculentum L. Journal of Applied Biology and Biotechnology, 4, 42–46.
Sun, Z., Copolovici, L., & Niinemets, Ü (2012). Can the capacity for isoprene emission acclimate to environmental modifications during autumn senescence in temperate deciduous tree species Populus tremula? Journal of Plant Research, 125(2), 263–274.
Sy, A., Timmers, A. C. J., Knief, C., & Vorholt, J. A. (2005). Methylotrophic metabolism is advantageous for Methylobacterium extorquens during colonization of Medicago truncatula under competitive conditions. Applied and Environmental Microbiology, 71(11), 7245–7252.
Talibart, R., Jebbar, M., Gouesbet, G., Himdi-Kabbab, S., Wróblewski, H., Blanco, C., & Bernard, T. (1994). Osmoadaptation in rhizobia: Ectoine-induced salt tolerance. Journal of Bacteriology, 176(17), 5210–5217. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC196703/.
Tani, A., Takai, Y., Suzukawa, I., Akita, M., Murase, H., & Kimbara, K. (2012). Practical application of methanol-mediated mutualistic symbiosis between Methylobacterium species and a roof greening moss, Racomitrium japonicum. PLoS ONE, 7(3), e33800. https://doi.org/10.1371/journal.pone.0033800.
Torres-Barceló, C., Cabot, G., Oliver, A., Buckling, A., & MacLean, R. C. (2013). A trade-off between oxidative stress resistance and DNA repair plays a role in the evolution of elevated mutation rates in bacteria. Proceedings of the Royal Society B: Biological Sciences, 280(1757), 20130007. https://doi.org/10.1098/rspb.2013.0007.
Truyens, S., Weyens, N., Cuypers, A., & Vangronsveld, J. (2015). Bacterial seed endophytes: Genera, vertical transmission and interaction with plants. Environmental Microbiology Reports, 7(1), 40–50. https://doi.org/10.1111/1758-2229.12181.
Tsuzuki, M., Moskvin, O. V., Kuribayashi, M., Sato, K., Retamal, S., Abo, M., et al. (2011). Salt stress-induced changes in the transcriptome, compatible solutes, and membrane lipids in the facultatively phototrophic bacterium Rhodobacter sphaeroides. Applied and Environmental Microbiology, 77(21), 7551–7559. https://doi.org/10.1128/AEM.05463-11.
Van Aken, B., Peres, C. M., Doty, S. L., Yoon, J. M., & Schnoor, J. L. (2004). Methylobacterium populi sp. nov., a novel aerobic, pink-pigmented, facultatively methylotrophic, methane-utilizing bacterium isolated from poplar trees (Populus deltoides × nigra DN34). International Journal of Systematic and Evolutionary Microbiology, 54(4), 1191–1196. https://doi.org/10.1099/ijs.0.02796-0.
Voigt, B., Schweder, T., Becher, D., Ehrenreich, A., Gottschalk, G., Feesche, J., Maurer, K. H., & Hecker, M. (2004). A proteomic view of cell physiology of Bacillus licheniformis. Proteomics, 4(5), 1465–1490.
Wagner, M. A., Eschenbrenner, M., Horn, T. A., Kraycer, J. A., Mujer, C. V., Hagius, S., et al. (2002). Global analysis of the Brucella melitensis proteome: Identification of proteins expressed in laboratory-grown culture. Proteomics, 2(8), 1047–1060.
Wasim, M., Bible, A. N., Xie, Z., & Alexandre, G. (2009). Alkyl hydroperoxide reductase has a role in oxidative stress resistance and in modulating changes in cell-surface properties in Azospirillum brasilense Sp245. Microbiology, 155(4), 1192–1202. https://doi.org/10.1099/mic.0.022541-0.
Whitaker, W. B., Sandoval, N. R., Bennett, R. K., Fast, A. G., & Papoutsakis, E. T. (2015). Synthetic methylotrophy: Engineering the production of biofuels and chemicals based on the biology of aerobic methanol utilization. Current Opinion in Biotechnology, 33, 165–175. https://doi.org/10.1016/j.copbio.2015.01.007.
Yaish, M. W., Antony, I., & Glick, B. R. (2015). Isolation and characterization of endophytic plant growth-promoting bacteria from date palm tree (Phoenix dactylifera L.) and their potential role in salinity tolerance. Antonie van Leeuwenhoek, 107(6), 1519–1532. https://doi.org/10.1007/s10482-015-0445-z.
Zaprasis, A., Brill, J., Thüring, M., Wünsche, G., Heun, M., Barzantny, H., et al. (2013). Osmoprotection of Bacillus subtilis through import and proteolysis of proline-containing peptides. Applied and Environmental Microbiology, 79(2), 576–587. https://doi.org/10.1128/AEM.01934-12.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B04035601).
Author information
Authors and Affiliations
Contributions
RS presented the idea, carried out the experiment, collected data and wrote the manuscript. ALK, MW and IU contributed to data analysis and manuscript drafting. SB, Y-HK, SA and S-MK helped in biochemical analysis. I-JL, as the team leader, supervised the design and conducting of all the experiments, and provided the relevant facilities, financial support, and mentorship. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Conflict of interest
All authors have no competing interests.
Ethics approval and consent to participate
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shahzad, R., Khan, A.L., Waqas, M. et al. Metabolic and proteomic alteration in phytohormone-producing endophytic Bacillus amyloliquefaciens RWL-1 during methanol utilization. Metabolomics 15, 16 (2019). https://doi.org/10.1007/s11306-018-1467-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-018-1467-0