Abstract
Introduction
The effects of exercise on the heart and its resistance to disease are well-documented. Recent studies have identified that exercise-induced resistance to arrhythmia is due to the preservation of mitochondrial membrane potential.
Objectives
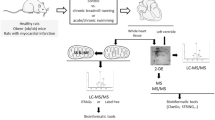
To identify novel metabolic changes that occur parallel to these mitochondrial alterations, we performed non-targeted metabolomics analysis on hearts from sedentary and exercise-trained rats challenged with isolated heart ischemia–reperfusion injury (I/R).
Methods
Eight-week old Sprague–Dawley rats were treadmill trained 5 days/week for 6 weeks (exercise duration and intensity progressively increased to 1 h at 30 m/min up a 10.5% incline, 75–80% VO2max). The recovery of pre-ischemic function for sedentary rat hearts was 28.8 ± 5.4% (N = 12) compared to exercise trained hearts, which recovered 51.9% ± 5.7 (N = 14) (p < 0.001).
Results
Non-targeted GC–MS metabolomics analysis of (1) sedentary rat hearts; (2) exercise-trained rat hearts; (3) sedentary rat hearts challenged with global ischemia–reperfusion (I/R) injury; and (4) exercise-trained rat hearts challenged with global I/R (10/group) revealed 15 statistically significant metabolites between groups by ANOVA using Metaboanalyst (p < 0.001). Enrichment analysis of these metabolites for pathway-associated metabolic sets indicated a > 10-fold enrichment for ammonia recycling and protein biosynthesis. Subsequent comparison of the sedentary hearts post-I/R and exercise-trained hearts post-I/R further identified significant differences in three metabolites (oleic acid, pantothenic acid, and campesterol) related to pantothenate and CoA biosynthesis (p ≤ 1.24E−05, FDR ≤ 5.07E−4).
Conclusions
These studies shed light on novel mechanisms in which exercise-induced cardioprotection occurs in I/R that complement both the mitochondrial stabilization and antioxidant mechanisms recently described. These findings also link protein synthesis and protein degradation (protein quality control mechanisms) with exercise-linked cardioprotection and mitochondrial susceptibility for the first time in cardiac I/R.







Similar content being viewed by others
Abbreviations
- AF:
-
Aortic flow
- CF:
-
Coronary flow
- CoA:
-
Coenzyme A
- CO:
-
Cardiac output
- I/R:
-
Ischemia/reperfusion
- PC:
-
Principal component
- PCA:
-
Principal components analysis
- SOD:
-
Superoxide dismutase
- SP:
-
Peak systolic pressure
References
Alleman, R. J., Tsang, A. M., Ryan, T. E., Patteson, D. J., Mcclung, J. M., Spangenburg, E. E., Shaikh, S. R., Neufer, P. D., & Brown, D. A. (2016). Exercise-induced protection against reperfusion arrhythmia involves stabilization of mitochondrial energetics. American Journal of Physiology Heart and Circulatory Physiology, 310, H1360–H1370.
Arsenian, M. (1998). Potential cardiovascular applications of glutamate, aspartate, and other amino acids. Clinical Cardiology, 21, 620–624.
Ascensao, A., Ferreira, R., & Magalhaes, J. (2007). Exercise-induced cardioprotection–biochemical, morphological and functional evidence in whole tissue and isolated mitochondria. International Journal of Cardiology, 117, 16–30.
Awad, A. B., Smith, A. J., & Fink, C. S. (2001). Plant sterols regulate rat vascular smooth muscle cell growth and prostacyclin release in culture. Prostaglandins Leukot Essent Fatty Acids, 64, 323–330.
Banerjee, R., Bultman, S. J., Holley, D., Hillhouse, C., Bain, J. R., Newgard, C. B., Muehlbauer, M. J., & Willis, M. S. (2015). Non-targeted metabolomics of Brg1/Brm double-mutant cardiomyocytes reveals a novel role for SWI/SNF complexes in metabolic homeostasis. Metabolomics, 11, 1287–1301.
Bolotin, G., Raman, J., Williams, U., Bacha, E., Kocherginsky, M., & Jeevanandam, V. (2007). Glutamine improves myocardial function following ischemia–reperfusion injury. Asian Cardiovascular and Thoracic Annals, 15, 463–467.
Boluyt, M. O., Brevick, J. L., Rogers, D. S., Randall, M. J., Scalia, A. F., & Li, Z. B. (2006). Changes in the rat heart proteome induced by exercise training: Increased abundance of heat shock protein HSP20. Proteomics, 6, 3154–3169.
Borges, J. P., & Lessa, M. A. (2015). mechanisms involved in exercise-induced cardioprotection: A systematic review. Arquivos Brasileiros de Cardiologia, 105, 71–81.
Bowles, D. K., Farrar, R. P., & Starnes, J. W. (1992). Exercise training improves cardiac function after ischemia in the isolated, working rat heart. American Journal of Physiology, 263, H804–H809.
Constantin-Teodosiu, D., Cederblad, G., & Hultman, E. (1991). A sensitive radioisotopic assay of pyruvate dehydrogenase complex in human muscle tissue. Analytical Biochemistry, 198, 347–351.
Dennis, S. C., Gevers, W., & Opie, L. H. (1991). Protons in ischemia: Where do they come from; where do they go to? Journal of Molecular and Cellular Cardiology, 23, 1077–1086.
Drake, K. J., Sidorov, V. Y., Mcguinness, O. P., Wasserman, D. H., & Wikswo, J. P. (2012). Amino acids as metabolic substrates during cardiac ischemia. Experimental Biology and Medicine (Maywood), 237, 1369–1378.
Dudley, G. A., Abraham, W. M., & Terjung, R. L. (1982). Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. Journal of Applied Physiology Respiratory, Environmental and Exercise Physiology, 53, 844–850.
Edwards, H. V., Scott, J. D., & Baillie, G. S. (2012). PKA phosphorylation of the small heat-shock protein HSP20 enhances its cardioprotective effects. Biochemical Society Transactions, 40, 210–214.
Fassbender, K., Lutjohann, D., Dik, M. G., Bremmer, M., Konig, J., Walter, S., Liu, Y., Letiembre, M., Von Bergmann, K., & Jonker, C. (2008). Moderately elevated plant sterol levels are associated with reduced cardiovascular risk–the LASA study. Atherosclerosis, 196, 283–288.
Finegold, J. A., Asaria, P., & Francis, D. P. 2013. Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations. International Journal of Cardiology, 168, 934–945.
Gailis, L., & Benmouyal, E. (1973). Endogenous alanine, glutamate, aspartate, and glutamine in the perfused guinea-pig heart: Effect of substrates and cardioactive agents. Canadian Journal of Biochemistry, 51, 11–20.
Huster, D., Reichenbach, A., & Reichelt, W. (2000). The glutathione content of retinal Muller (glial) cells: Effect of pathological conditions. Neurochemistry International, 36, 461–469.
Jennings, R. B., & Reimer, K. A. (1991). The cell biology of acute myocardial ischemia. Annual Review of Medicine, 42, 225–246.
Koone, M. D., Rizzo, W. B., Elias, P. M., Williams, M. L., Lightner, V., & Pinnell, S. R. (1990). Ichthyosis, mental retardation, and asymptomatic spasticity. A new neurocutaneous syndrome with normal fatty alcohol:NAD+ oxidoreductase activity. Archives of Dermatology, 126, 1485–1490.
Kumerova, A. O., Utno, L., Lipsberga, Z. E., & Shkestere, I. (1992). [Study of pantothenic acid derivatives as cardiac protectors in a model of experimental ischemia and reperfusion of the isolated heart]. Biulleten Eksperimentalnoi Biologii I Meditsiny, 113, 373–375.
Lee, Y., Min, K., Talbert, E. E., Kavazis, A. N., Smuder, A. J., Willis, W. T., & Powers, S. K. (2012). Exercise protects cardiac mitochondria against ischemia–reperfusion injury. Medicine and Science in Sports and Exercise, 44, 397–405.
Liu, J., Marchase, R. B., & Chatham, J. C. (2007). Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. Journal of Molecular and Cellular Cardiology, 42, 177–185.
Liu, Y. T., Jia, H. M., Chang, X., Ding, G., Zhang, H. W., & ZOU, Z. M. (2013). The metabolic disturbances of isoproterenol induced myocardial infarction in rats based on a tissue targeted metabonomics. Molecular BioSystems, 9, 2823–2834.
Locke, M., Noble, E. G., Tanguay, R. M., Feild, M. R., Ianuzzo, S. E., & Ianuzzo, C. D. (1995). Activation of heat-shock transcription factor in rat heart after heat shock and exercise. American Journal of Physiology, 268, C1387–C1394.
Melling, C. W., Thorp, D. B., & Noble, E. G. (2004). Regulation of myocardial heat shock protein 70 gene expression following exercise. Journal of Molecular and Cellular Cardiology, 37, 847–855.
Miller-Graber, P., Lawrence, L., Fisher, M., Bump, K., Foreman, J., & Kurcz, E. (1991). Metabolic responses to ammonium acetate infusion in exercising horses. The Cornell Veterinarian, 81, 397–410.
Mitchell, C. R., Harris, M. B., Cordaro, A. R., & Starnes, J. W. (2002). Effect of body temperature during exercise on skeletal muscle cytochrome c oxidase content. Journal of Applied Physiology(1985), 93, 526–530.
Moran, A. E., Forouzanfar, M. H., Roth, G. A., Mensah, G. A., Ezzati, M., Murray, C. J., & Naghavi, M. (2014). Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: The Global Burden of Disease 2010 study. Circulation, 129, 1483–1492.
Musman, J., Pons, S., Barau, C., Caccia, C., Leoni, V., Berdeaux, A., Ghaleh, B., & Morin, D. (2016). Regular treadmill exercise inhibits mitochondrial accumulation of cholesterol and oxysterols during myocardial ischemia–reperfusion in wild-type and ob/ob mice. Free Radical Biology & Medicine, 101, 317–324.
Nowbar, A. N., Howard, J. P., Finegold, J. A., Asaria, P., & Francis, D. P. (2014). 2014 global geographic analysis of mortality from ischaemic heart disease by country, age and income: Statistics from World Health Organisation and United Nations. International Journal of Cardiology, 174, 293–298.
Palomer, X., Barroso, E., Zarei, M., Botteri, G., & Vazquez-Carrera, M (2016). PPARbeta/delta and lipid metabolism in the heart. Biochim Biophys Acta, 1861, 1569–1578.
Park, J. W., Chun, Y. S., Kim, M. S., Park, Y. C., Kwak, S. J., & Park, S. C. (1998). Metabolic modulation of cellular redox potential can improve cardiac recovery from ischemia–reperfusion injury. International Journal of Cardiology, 65, 139–147.
Paroo, Z., Meredith, M. J., Locke, M., Haist, J. V., Karmazyn, M., & Noble, E. G. (2002). Redox signaling of cardiac HSF1 DNA binding. American Journal of Physiology Cell Physiology, 283, C404–C411.
Powers, S. K., Lennon, S. L., Quindry, J., & Mehta, J. L. (2002). Exercise and cardioprotection. Current Opinion in Cardiology, 17, 495–502.
Powers, S. K., Quindry, J. C., & Kavazis, A. N. (2008). Exercise-induced cardioprotection against myocardial ischemia–reperfusion injury. Free Radical Biology & Medicine, 44, 193–201.
Powers, S. K., Sollanek, K. J., Wiggs, M. P., Demirel, H. A., & Smuder, A. J. (2014). Exercise-induced improvements in myocardial antioxidant capacity: The antioxidant players and cardioprotection. Free Radical Research, 48, 43–51.
Rau, E. E., Shine, K. I., Gervais, A., Douglas, A. M., & Amos, E. C. 3rd (1979). Enhanced mechanical recovery of anoxic and ischemic myocardium by amino acid perfusion. American Journal of Physiology, 236, H873–H879.
Reeves, P. G., Nielsen, F. H., & Fahey, G. C., Jr. (1993). AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. Journal of Nutrition, 123, 1939–1951.
Rennie, M. J., Bowtell, J. L., Bruce, M., & Khogali, S. E. (2001). Interaction between glutamine availability and metabolism of glycogen, tricarboxylic acid cycle intermediates and glutathione. Journal of Nutrition, 131, 2488S–2490S. (Discussion 2496S–2497S).
Rizzo, W. B., Craft, D. A., Judd, L. L., Moser, H. W., & Moser, A. B. (1993). Fatty alcohol accumulation in the autosomal recessive form of rhizomelic chondrodysplasia punctata. Biochemical Medicine and Metabolic Biology, 50, 93–102.
Robinet, A., Hoizey, G., & Millart, H. (2005). PI 3-kinase, protein kinase C, and protein kinase A are involved in the trigger phase of beta1-adrenergic preconditioning. Cardiovascular Research, 66, 530–542.
Saggerson, E. D., & Greenbaum, A. L. (1970). The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue. Biochemistry Journal, 119, 193–219.
Stottrup, N. B., Kristiansen, S. B., Lofgren, B., Hansen, B. F., Kimose, H. H., Botker, H. E., & Nielsen, T. T. (2006). l-glutamate and glutamine improve haemodynamic function and restore myocardial glycogen content during postischaemic reperfusion: A radioactive tracer study in the rat isolated heart. Clinical and Experimental Pharmacology and Physiology, 33, 1099–1103.
Sud, M., Fahy, E., Cotter, D., Azam, K., Vadivelu, I., Burant, C., Edison, A., Fiehn, O., Higashi, R., Nair, K. S., Sumner, S., & Subramaniam, S. (2016). Metabolomics Workbench: An international repository for metabolomics data and metadata, metabolite standards, protocols, tutorials and training, and analysis tools. Nucleic Acids Research, 44, D463–D470.
Taegtmeyer, H., Peterson, M. B., Ragavan, V. V., Ferguson, A. G., & Lesch, M. (1977). De novo alanine synthesis in isolated oxygen-deprived rabbit myocardium. Journal of Biological Chemistry, 252, 5010–5018.
Tuunanen, H., & Knuuti, J. (2011). Metabolic remodelling in human heart failure. Cardiovascular Research, 90, 251–257.
Wischmeyer, P. E., Jayakar, D., Williams, U., Singleton, K. D., Riehm, J., Bacha, E. A., Jeevanandam, V., Christians, U., & Serkova, N. (2003). Single dose of glutamine enhances myocardial tissue metabolism, glutathione content, and improves myocardial function after ischemia–reperfusion injury. JPEN Journal of Parenteral and Enteral Nutrition, 27, 396–403.
Wolk, A., Vessby, B., Ljung, H., & Barrefors, P. (1998). Evaluation of a biological marker of dairy fat intake. American Journal of Clinical Nutrition, 68, 291–295.
Wong, H. S., Chen, N., Leong, P. K., & Ko, K. M. (2014). beta-Sitosterol enhances cellular glutathione redox cycling by reactive oxygen species generated from mitochondrial respiration: Protection against oxidant injury in H9c2 cells and rat hearts. Phytotherapy Research, 28, 999–1006.
Xia, J., Psychogios, N., Young, N., & Wishart, D. S. (2009). MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Research, 37, W652–W660.
Xia, J., Sinelnikov, I. V., Han, B., & Wishart, D. S. (2015). MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Research, 43, W251–W257.
Zhang, S., Li, H., & Yang, S. J. (2010). Tribulosin protects rat hearts from ischemia/reperfusion injury. Acta Pharmacologica Sinica, 31, 671–678.
Acknowledgements
This work was supported by the National Institutes of Health (R01HL104129 to MSW), the Leducq Foundation Transatlantic Networks of Excellence (11CVD04 to MSW and CP), and the American Heart Association (Post-Doctoral Fellowship to TP).
Author information
Authors and Affiliations
Contributions
TP, JS, and MW conceived and designed the experiments; TP, JS, AH, PH, JB, MM, and AI performed the experiments, SO, JB, MM, and MW were involved in the analysis and interpretation of the data, and JS, TP, and MW wrote the draft manuscript. TP, JS, SO, JB, MM, AH, AI, PC, CP, and MW edited and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This investigation was approved by the UNC-Greensboro’s Animal Care and Use Committee and conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85–23, Revised 1996). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11306_2017_1303_MOESM1_ESM.pdf
Supplemental Fig. 1. Heatmap analysis of all named metabolites from all individual rat hearts from all four groups (exercise trained, exercise trained post-ischemia reperfusion, sedentary, sedentary post-ischemia reperfusion) included in the ANOVA analysis of significance. N = 10 biological replicates/group.
Supplemental Fig. 2. Heatmap analysis of all named metabolites from all individual rat hearts included in the t test analysis of significance (sedentary I/R vs. exercise trained I/R). N = 10 biological replicates/group.
Supplemental Fig. 3. Peak value comparisons of ANOVA significant metabolites involved in the citric acid cycle in sedentary and exercise trained hearts ± ischemia reperfusion injury. Peak values of (A) Glucose (and other aldohexoses), and (B) Glucose-6-phosphate from rat hearts after sedentary, exercise trained, sedentary ischemia reperfusion injury, and exercise trained reperfusion injury. An ANOVA was run to determine significance, followed by a post-hoc Fisher’s Least Significant Difference (LSD) multiple comparison between groups. N = 10 biological replicates/group. *p < 0.05 versus to I/R, **p < 0.05 versus exercise,#p < 0.05 versus exercise I/R. Data is presented as the mean ± SEM.
Spplemental Fig. 4. Peak value comparisons of t test significant metabolites identified by comparing sedentary I/R and exercise trained I/R. Peak values of (A) Oleic acid, (B) Pantothenic acid, and (C) Campesterol from rat hearts after sedentary, exercise trained, sedentary ischemia reperfusion injury, and exercise trained reperfusion injury. Although these metabolites were identified by t test significance, this graph presents their ANOVA statistics from the initial analysis (i.e., both t test and ANOVA significant). An ANOVA was run to determine significance, followed by a post-hoc Fisher’s Least Significant Difference (LSD) multiple comparison between groups. N = 10 biological replicates/group. *p < 0.05 versus to I/R, **p < 0.05 versus exercise, #p < 0.05 versus exercise I/R. Data is presented as the mean ± SEM.
Supplemental Fig. 5. Enrichment analysis of t test significant metabolites comparing sedentary I/R versus exercise trained I/R. Enrichment analysis was performed using Metaboanalyst, comparing to (A) Pathway associated metabolite sets, (B) Predicted Metabolite sets, and (C) Enrichment of Location-based metabolite sets. (PDF 1586 KB)
Rights and permissions
About this article
Cite this article
Parry, T.L., Starnes, J.W., O’Neal, S.K. et al. Untargeted metabolomics analysis of ischemia–reperfusion-injured hearts ex vivo from sedentary and exercise-trained rats. Metabolomics 14, 8 (2018). https://doi.org/10.1007/s11306-017-1303-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-017-1303-y