Abstract
Introduction
Human plasma metabolomics offer powerful tools for understanding disease mechanisms and identifying clinical biomarkers for diagnosis, efficacy prediction and patient stratification. Although storage conditions can affect the reliability of data from metabolites, strict control of these conditions remains challenging, particularly when clinical samples are included from multiple centers. Therefore, it is necessary to consider stability profiles of each analyte.
Objectives
The purpose of this study was to extract unstable metabolites from vast metabolome data and identify factors that cause instability.
Method
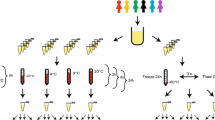
Plasma samples were obtained from five healthy volunteers, were stored under ten different conditions of time and temperature and were quantified using leading-edge metabolomics. Instability was evaluated by comparing quantitation values under each storage condition with those obtained after −80 °C storage.
Result
Stability profiling of the 992 metabolites showed time- and temperature-dependent increases in numbers of significantly changed metabolites. This large volume of data enabled comparisons of unstable metabolites with their related molecules and allowed identification of causative factors, including compound-specific enzymatic activity in plasma and chemical reactivity. Furthermore, these analyses indicated extreme instability of 1-docosahexaenoylglycerol, 1-arachidonoylglycerophosphate, cystine, cysteine and N6-methyladenosine.
Conclusion
A large volume of data regarding storage stability was obtained. These data are a contribution to the discovery of biomarker candidates without misselection based on unreliable values and to the establishment of suitable handling procedures for targeted biomarker quantification.





Similar content being viewed by others
Abbreviations
- BMI:
-
Body mass index
- C3f:
-
Complement
- EPA:
-
Eicosapentaenoic acid
- FDR:
-
False discovery rate
- GC/MS:
-
Gas chromatography/mass spectrometry
- GluTrp:
-
Glutamyltryptophan
- GPC:
-
Glycerophosphorylcholine
- GPE:
-
Glycerophosphoethanolamine
- LPC:
-
Lysophosphatidylcholine
- LPE:
-
Lysophophatidylethanolamine
- LPI:
-
Lysophosphatidylinositol
- MAG:
-
Monoacylglycerol
- RNase:
-
Ribonucleotidase
- ROS:
-
Reactive oxygen species
- PC:
-
Phosphorylcholine
- PCA:
-
Principal component analysis
- RT:
-
Room temperature
- SOP:
-
Standard operation procedure
- TAG:
-
Triacylglycerol
- UHPLC/MS/MS:
-
Ultrahigh performance liquid chromatography/tandem mass spectrometry
References
Agarwal, S., Vargas, G., Nordstrom, C., Tam, E., Buffone, G. J., & Devaraj, S. (2015). Effect of interference from hemolysis, icterus and lipemia on routine pediatric clinical chemistry assays. Clinica Chimica Acta, 438, 241–245.
Ames, B. N., Cathcart, R., Schwiers, E., & Hochstein, P. (1981). Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proceedings of the National Academy of Sciences, 78, 6858–6862.
Davies, S. K., Ang, J. E., Revell, V. L., Holmes, B., Mann, A., Robertson, F. P., et al. (2014). Effect of sleep deprivation on the human metabolome. Proceedings of the National Academy of Sciences, 111, 10761–10766.
Elin, C., Michael, B. S., Thomas, M., & Henrik, A. (2012). Physical fitness level is reflected by alterations in the human plasma metabolome. Molecular BioSystems, 8, 1187–1196.
Evans, A. M., Bridgewater, B. R., Miller, L. A. D., Mitchell, M. W., Robinson, R. J., Dai, H., et al. (2014). High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics, 4(2), 1.
Evans, A. M., DeHaven, C. D., Barrett, T., Mitchell, M., & Milgram, E. (2009). Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Analytical Chemistry, 81, 6656–6667.
Fiskerstrand, T., Refsum, H., Kvalheim, G., & Ueland, P. M. (1993). Homocysteine and other thiols in plasma and urine: Automated determination and sample stability. Clinical Chemistry, 39, 263–271.
Fliniaux, O., Gaillard, G., Lion, A., Cailleu, D., Mesnard, F., Betsou, F., et al. (2011). Influence of common preanalytical variations on the metabolic profile of serum samples in biobanks. Journal of Biomolecular NMR, 51, 457–465.
International Expert Committee. (2009). International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care, 32, 1327–1334.
Kamlage, B., Maldonado, S. G., Bethan, B., Peter, E., Schmitz, O., Liebenberg, V., et al. (2014). Quality markers addressing preanalytical variations of blood and plasma processing identified by broad and targeted metabolite profiling. Clinical Chemistry, 60, 399–412.
Kand’ar, R., Zakova, P., & Muzakova, V. (2006). Monitoring of antioxidant properties of uric acid in humans for a consideration measuring of levels of allantoin in plasma by liquid chromatography. Clinica Chimica Acta, 365, 249–256.
Kasukawa, T., Sugimoto, M., Hida, A., Minami, Y., Mori, M., Honma, S., et al. (2012). Human blood metabolite timetable indicates internal body time. Proceedings of the National Academy of Sciences United States of America, 109, 15036–15041.
Kate, D. M., & Samie, R. J. (2014). The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nature Reviews Molecular Cell Biology, 15, 313–326.
Kleinman, W. A., & Richie, J. P., Jr. (2000). Status of glutathione and other thiols and disulfides in human plasma. Biochemical Pharmacology, 60, 19–29.
Krug, S., Kastenmüller, G., Stückler, F., Rist, M. J., Skurk, T., Sailer, M., et al. (2012). The dynamic range of the human metabolome revealed by challenges. FASEB Journal, 26, 2607–2619.
Mollnes, T., Garred, P., & Bergseth, G. (1988). Effect of time, temperature and anticoagulants on in vitro complement activation: Consequences for collection and preservation of samples to be examined for complement activation. Clinical and Experimental Immunology, 73, 484–486.
Ohta, T., Masutomi, N., Tsutsui, N., Sakairi, T., Mitchell, M., Milburn, M. V., et al. (2009). Untargeted metabolomic profiling as an evaluative tool of fenofibrate-induced toxicology in Fischer 344 male rats. Toxicologic Pathology, 37, 521–535.
Pastor, A., Farre, M., Fito, M., Fernandez-Aranda, F., & de la Torre, R. (2014). Analysis of ECs and related compounds in plasma: Artifactual isomerization and ex vivo enzymatic generation of 2-MGs. Journal of Lipid Research, 55, 966–977.
Pellis, L., van Erk, M. J., van Ommen, B., Bakker, G. C., Hendriks, H. F., Cnubben, N. H., et al. (2012). Plasma metabolomics and proteomics profiling after a postprandial challenge reveal subtle diet effects on human metabolic status. Metabolomics, 8, 347–359.
Pinto, J., Domingues, M. R., Galhano, E., Pita, C., Almeida, M. C., Carreira, I. M., et al. (2014). Human plasma stability during handling and storage: Impact on NMR metabolomics. Analyst, 139, 1168–1177.
Storey, J. D., & Tibshirani, R. (2003). Statistical significance for genome wide studies. Proceedings of the National Academy of Sciences United States of America, 100, 9440–9445.
Teahan, O., Gamble, S., Holmes, E., Waxman, J., Nicholson, J. K., Bevan, C., et al. (2006). Impact of analytical bias in metabonomic studies of human blood serum and plasma. Analytical Chemistry, 78, 4307–4318.
Wu, X. W., Lee, C. C., Muzny, D. M., & Caskey, C. T. (1989). Urate oxidase: Primary structure and evolutionary implications. Proceedings of the National Academy of Sciences United States of America, 86, 9412–9416.
Yin, P., Lehmann, R., & Xu, G. (2015). Effects of pre-analytical processes on blood samples used in metabolomics studies. Analytical and Bioanalytical Chemistry, 407, 4879–4892.
Yin, P., Peter, A., Franken, H., Zhao, X., Neukamm, S. S., Rosenbaum, L., et al. (2013). Preanalytical aspects and sample quality assessment in metabolomics studies of human blood. Clinical Chemistry, 59, 833–845.
Yu, Z., Zhai, G., Singmann, P., He, Y., Xu, T., Prehn, C., et al. (2012). Human serum metabolic profiles are age dependent. Aging Cell, 11, 960–967.
Zighetti, M. L., Chantarangkul, V., Lombardi, R., Lecchi, A., & Cattaneo, M. (2004). Effects of some pre-analytical conditions on the measurement of homocysteine and cysteine in plasma. Clinical Chemistry and Laboratory Medicine, 42, 204–207.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors ‘Takeo Moriya, Yoshinori Satomi and Hiroyuki Kobayashi’ declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moriya, T., Satomi, Y. & Kobayashi, H. Intensive determination of storage condition effects on human plasma metabolomics. Metabolomics 12, 179 (2016). https://doi.org/10.1007/s11306-016-1126-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-016-1126-2