Abstract
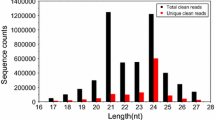
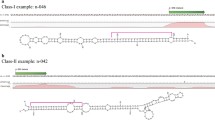
microRNAs (miRNAs) play vital roles in plants regulating a panoply of biological processes, such as development, hormone signaling, and stress response, by inhibiting target genes at the post-transcriptional level. However, the roles of miRNAs in Betula luminifera remain elusive. To mine for B. luminifera miRNAs and targets, we used a deep sequencing approach to analyze the sRNAs and degradome sequencing of mixed samples, including roots, stems, and leaves. A total of 114 known miRNAs or miRNA*s from 44 families, and 24 novel miRNAs and 17 miRNA*s plus 15 plausible miRNA candidates were identified, of which 36 known miRNAs, 29 miRNA*s, and all novel miRNAs had precursor sequences. Additionally, 49 targets for 19 known miRNA families and seven miRNA*s, and seven targets for novel miRNAs were identified using a high-throughput degradome-sequencing approach. The conserved miRNA targets were mainly transcription factors, whereas the miRNA* targets were mainly protein-coding genes, with preferential propensity to functional enzymes. A Gene Ontology analysis showed that the predicted targets were classified into 62 biological processes, 20 cellular components, and 28 molecular functions, respectively. We found two different targets for miR396a* and miR396c*, and the target changed when the miR156d precursor cleavage site was shifted toward the 5′-end by two nucleotides, indicating the diverse regulatory roles of MIRNA genes. Furthermore, three targets identified by degradome sequencing were validated further through 5′ rapid amplification of cDNA ends. The expression patterns of the randomly selected miRNAs varied among different tissues. miR164 expression was induced under nitrogen starvation, with tissue-specific expression patterns, and was negatively correlated with the NAC1 target gene in roots and leaves, but not in stems. This study is a transcriptome-based analysis of miRNAs and the degradome, providing useful information to explore the functions of miRNAs and their target genes in B. luminifera.






Similar content being viewed by others
References
Addo-Quaye C, Eshoo TW, Bartel DP, Axtell MJ (2008) Endogenous siRNA and miRNA targets identified by sequencing of the Arabidopsis degradome. Curr Biol 18:758–762
Alonso-Peral MM, Li J, Li Y, Allen RS, Schnippenkoetter W, Ohms S, White RL, Millar AA (2010) The miR159 regulated GAMYB-like genes inhibit growth and promote programmed cell death in Arabidopsis. Plant Physiol. doi:10.1104/pp.110.160630
Ashburner M, Bergman CM (2005) Drosophila melanogaster: a case study of a model genomic sequence and its consequences. Genome Res 15:1661–1667
Axtell MJ, Bowman JL (2008) Evolution of plant microRNAs and their targets. Trends Plant Sci 13:343–349
Bonnet E, He Y, Billiau K, Van de Peer Y (2010) TAPIR, a web server for the prediction of plant microRNA targets, including target mimics. Bioinformatics 26:1566–1568
Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L, Voinnet O (2008) Widespread translational inhibition by plant miRNAs and siRNAs. Science 320:1185–1190
Carthew RW, Sontheimer EJ (2009) Origins and mechanisms of miRNAs and siRNAs. Cell 136:642–655
Chuck G, Candela H, Hake S (2009) Big impacts by small RNAs in plant development. Curr Opin Plant Biol 12:81–86
Constabel CP, Yip L, Patton JJ, Christopher ME (2000) Polyphenol oxidase from hybrid poplar. Cloning and expression in response to wounding and herbivory. Plant Physiol 124:285–295
Czech B, Zhou R, Erlich Y, Brennecke J, Binari R, Villalta C, Gordon A, Perrimon N, Hannon GJ (2009) Hierarchical rules for Argonaute loading in Drosophila. Mol Cell 36:445–456
Dai X, Zhao PX (2011) psRNATarget: a plant small RNA target analysis server. Nucleic Acids Res. doi:10.1093/nar/GKR319:1-5
De Paola D, Cattonaro F, Pignone D, Sonnante G (2012) The miRNAome of globe artichoke: conserved and novel micro RNAs and target analysis. BMC Genomics 13:41
Du Z, Zhou X, Ling Y, Zhang Z, Su Z (2010) agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res 38(Web Server issue):W64–W70
Fahlgren N, Howell MD, Kasschau KD, Chapman EJ, Sullivan CM, Cumbie JS, Givan SA, Law TF, Grant SR, Dangl JL, Carrington JC (2007) High-throughput sequencing of Arabidopsis microRNAs: evidence for frequent birth and death of MIRNA genes. PLoS One 2:e219
German MA, Pillay M, Jeong DH, Hetawal A, Luo S, Janardhanan P, Kannan V, Rymarquis LA, Nobuta K, German R, De Paoli E, Lu C, Schroth G, Meyers BC, Green PJ (2008) Global identification of microRNA-target RNA pairs by parallel analysis of RNA ends. Nat Biotechnol 26:941–946
Griffiths-Jones S, Hui JH, Marco A, Ronshaugen M (2011) MicroRNA evolution by arm switching. EMBO Rep 12:172–177
Guo HS, Xie Q, Fei JF, Chua NH (2005) MicroRNA directs mRNA cleavage of the transcription factor NAC1 to downregulate auxin signals for Arabidopsis lateral root development. Plant Cell 17:1376–1386
Hao DC, Yang L, Xiao PG, Liu M (2012) Identification of Taxus microRNAs and their targets with high-throughput sequencing and degradome analysis. Physiol Plant 146:388–403
Hsieh LC, Lin SI, Shih AC, Chen JW, Lin WY, Tseng CY, Li WH, Chiou TJ (2009) Uncovering small RNA-mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiol 151:2120–2132
Huang HH, Jiang C, Tong ZK, Cheng LJ, Zhu MY, Lin EP (2014) Eight distinct cellulose synthase catalytic subunit genes from Betula luminifera are associated with primary and secondary cell wall biosynthesis. Cellulose 21:2183–2198
Jiang J, Lv M, Liang Y, Ma Z, Cao J (2014) Identification of novel and conserved miRNAs involved in pollen development in Brassica campestris ssp. chinensis by high-throughput sequencing and degradome analysis. BMC Genomics 15:146
Jones-Rhoades MW, Bartel DP (2004) Computational identification of plant MicroRNAs and their targets, including a stress-induced miRNA. Mol Cell 14:787–799
Jones-Rhoades MW, Bartel DP, Bartel B (2006) MicroRNAs and their regulatory roles in plants. Annu Rev Plant Biol 57:19–53
Kozomara A, Griffiths-Jones S (2013) miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res:gkt1181
Kuang KR, Li PX (1979) Flora Republicae Popularis Sinicae. Beijing: Science Press 21:44–45
Li M, Xia Y, Gu Y, Zhang K, Lang Q, Chen L, Guan J, Luo Z, Chen H, Li Y, Li Q, Li X, Jiang AA, Shuai S, Wang J, Zhu Q, Zhou X, Gao X (2010a) MicroRNAome of porcine pre- and postnatal development. PLoS One 5:e11541
Li YF, Zheng Y, Addo-Quaye C, Zhang L, Saini A, Jagadeeswaran G, Axtell MJ, Zhang W, Sunkar R (2010b) Transcriptome-wide identification of microRNA targets in rice. Plant J 62:742–759
Li B, Qin Y, Duan H, Yin W, Xia X (2011) Genome-wide characterization of new and drought stress responsive microRNAs in Populus euphratica. J Exp Bot 62:3765–3779
Liu HJ, Qin C, Chen Z, Zuo T, Yang XR, Zhou HK, Xu M, Cao SL, Shen YO, Lin HJ, He XJ, Zhang YC, Li LJ, Ding HP, Lübberstedt T, Zhang ZM, Pan GT (2014) Identification of miRNAs and their target genes in developing maize ears by combined small RNA and degradome sequencing. BMC Genomics 15:25
Ma Z, Coruh C, Axtell MJ (2010) Arabidopsis lyrata Small RNAs: transient MIRNA and small interfering RNA loci within the Arabidopsis genus. Plant Cell 22:1090–1103
Mao W, Li Z, Xia X, Li Y, Yu J (2012) A combined approach of high-throughput sequencing and degradome analysis reveals tissue specific expression of microRNAs and their targets in cucumber. PLoS One 7:e33040
Matzke M, Kanno T, Daxinger L, Huettel B, Matzke AJ (2009) RNA-mediated chromatin-based silencing in plants. Curr Opin Cell Biol 21:367–376
Meyers BC, Axtell MJ, Bartel B, Bartel DP, Baulcombe D, Bowman JL, Cao X, Carrington JC, Chen X, Green PJ, Griffiths-Jones S, Jacobsen SE, Mallory AC, Martienssen RA, Poethig RS, Qi Y, Vaucheret H, Voinnet O, Watanabe Y, Weigel D, Zhu JK (2008) Criteria for annotation of plant MicroRNAs. Plant Cell 20:3186–3190
Morita-Yamamuro C, Tsutsui T, Sato M, Yoshioka H, Tamaoki M, Ogawa D, Matsuura H, Yoshihara T, Ikeda A, Uyeda I, Yamaguchi J (2005) The Arabidopsis gene CAD1 controls programmed cell death in the plant immune system and encodes a protein containing a MACPF domain. Plant Cell Physiol 46:902–912
Okamura K, Phillips MD, Tyler DM, Duan H, Chou YT, Lai EC (2008) The regulatory activity of microRNA* species has substantial influence on microRNA and 3′ UTR evolution. Nat Struct Mol Biol 15:354–363
Sablok G, Srivastva AK, Suprasanna P, Baev V, Ralph PJ (2015) isomiRs: increasing evidences of isomiRs complexity in plant stress functional biology. Front. Plant Sci 6:949
Shukla LI, Chinnusamy V, Sunkar R (2008) The role of microRNAs and other endogenous small RNAs in plant stress responses. Biochim Biophys Acta 1779:743–748
Sunkar R, Girke T, Jain PK, Zhu JK (2005) Cloning and characterization of microRNAs from rice. Plant Cell 17:1397–1411
Tedder P, Zubko E, Westhead DR, Meyer P (2009) Small RNA analysis in Petunia hybrida identifies unusual tissue-specific expression patterns of conserved miRNAs and of a 24mer RNA. RNA 15:1012–1020
Wu G, Park MY, Conway SR, Wang JW, Weigel D, Poethig RS (2009) The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138:750–759
Xie F, Wang Q, Zhang B (2015) Global microRNA modification in cotton (Gossypium hirsutum L.). Plant Biotechnol J 13:492–500
Yang J, Liu X, Xu B, Zhao N, Yang X, Zhang M (2013) Identification of miRNAs and their targets using high-throughput sequencing and degradome analysis in cytoplasmic male-sterile and its maintainer fertile lines of Brassica juncea. BMC Genomics 14:9
Zhang W, Luo Y, Gong X, Zeng W, Li S (2009) Computational identification of 48 potato microRNAs and their targets. Comput Biol Chem 33:84–93
Zhang Z, Yu J, Li D, Liu F, Zhou X, Wang T, Ling Y, Su Z (2010) PMRD: plant microRNA database. Nucleic Acids Res 38(Database issue):D806–D813
Zhang X, Zhao H, Gao S, Wang WC, Katiyar-Agarwal S, Huang HD, Raikhel N, Jin H (2011) Arabidopsis Argonaute 2 regulates innate immunity via miRNA393( *)-mediated silencing of a Golgi-localized SNARE gene, MEMB12. Mol Cell 42:356–366
Zhang J, Zhang S, Han S, Wu T, Li X, Li W, Qi L (2012) Genome-wide identification of microRNAs in larch and stage-specific modulation of 11 conserved microRNAs and their targets during somatic embryogenesis. Planta 236:647–657
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 31300566) and Zhejiang Province Science and Technology Support Program (No. 2012C12908-8).
Author contributions
JZ, MH and ZT conceived and designed the experiments, and wrote the paper. MH, JL, LC and JW performed the experiments, JZ and ZT analyzed the data, YP and LC contributed reagents/materials/ analytical tools. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Data archive statement
The sequencing data is currently being submitted to NCBI’s Gene Expression Omnibus (GEO, accession numbers: GSE80074), and the accession numbers will be supplied once available.
Additional information
Communicated by M. Troggio
Junhong Zhang and Menghui Huang contributed equally to this work.
Electronic supplementary material
Supplementary Table 1
5′ RACE Primers for validation of miRNA targets (DOC 29 kb)
Supplementary Table 2
miRNA primers used in qRT-PCR (DOC 34 kb)
Supplementary Table 3
The miRNA precursor sequences in B. luminifera (XLS 44 kb)
Supplementary Table 4
Identification of known miRNAs ambiguously from B. luminifera (DOC 81 kb)
Supplementary Table 5
Isolation and identification of candidate miRNAs in B. luminifera (DOC 46 kb)
Rights and permissions
About this article
Cite this article
Zhang, J., Huang, M., Liang, J. et al. Genome-wide mining for microRNAs and their targets in Betula luminifera using high-throughput sequencing and degradome analyses. Tree Genetics & Genomes 12, 99 (2016). https://doi.org/10.1007/s11295-016-1047-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11295-016-1047-2