Abstract
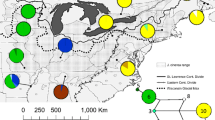
We examined the phylogeography of three south-east Australian trees (Eucalyptus delegatensis, Eucalyptus obliqua, and Eucalyptus regnans) with different tolerances, in terms of cold, drought, fire and soil to explore whether species with different ecologies share major phylogeographic patterns. A second aim of this study was to examine geographic patterns of chloroplast DNA (cpDNA) haplotype sharing among the three study species. Trees of E. delegatensis (n = 120), E. obliqua (n = 265) and E. regnans (n = 270) were genotyped with five cpDNA microsatellite markers. The species shared major phylogeographic disjunctions, and common patterns at proposed glacial refugia (generally high haplotype diversity) and areas thought to have been treeless during the Last Glacial Maximum (LGM) (low diversity). Inter-specific sharing of haplotypes was extensive, and fixation of shared, regional haplotypes was more frequent in areas postulated as having been treeless at the LGM. Despite ecological differences, chloroplast microsatellite data suggest the three species have responded to past climatic changes in a similar way, by persisting in multiple, generally common refugia. We suggest that the natural ability of eucalypt species to hybridise with others with quite different or broader ecological tolerances may provide an “insurance policy” for response to rapidly changing abiotic conditions.




Similar content being viewed by others
References
Acosta MC, Premoli AC (2010) Evidence of chloroplast capture in South American Nothofagus (subgenus Nothofagus, Nothofagaceae). Mol Phylogenet Evol 54:235–242
Allendorf FW, Luikart G (2007) Conservation and the genetics of populations. Blackwell Publishers, Oxford
Arnold M (1997) Natural hybridization and evolution. Oxford University Press, New York
Ashton D (1958) The ecology of Eucalyptus regnans F. Muell.: the species and its frost resistance. Aust J Bot 6:154–176
Ashton D (1981) The ecology of the boundary between Eucalyptus regnans F. Muell. and E. obliqua L'Herit. in Victoria. Proc Ecol Soc Aust 11:75–94
Bandelt HJ, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48
Beheregaray LB (2008) Twenty years of phylogeography: the state of the field and the challenges for the Southern Hemisphere. Mol Ecol 17:3754–3774
Belahbib N, Pemonge MH, Ouassou A, Sbay H, Kremer A, Petit RJ (2001) Frequent cytoplasmic exchanges between oak species that are not closely related: Quercus suber and Q. ilex in Morocco. Mol Ecol 10:2003–2012
Bloomfield JA, Nevill P, Potts BM, Vaillancourt RE, Steane DA (2011) Molecular genetic variation in a widespread forest tree species Eucalyptus obliqua (Myrtaceae) on the island of Tasmania. Aust J Bot 59:226–237
Boland D, Brooker M, Chippendale G, Hall N, Hyland B, Johnston R, Kleinig D, McDonald M, Turner J (2006) Forest trees of Australia, 5th edn. CSIRO Publishing, Melbourne
Bowman DM, Murphy BP, Neyland DL, Williamson GJ, Prior LD (2014) Abrupt fire regime change may cause landscape‐wide loss of mature obligate seeder forests. Glob Chang Biol 20:1008–1015
Brooker MIH (2000) A new classification of the genus Eucalyptus L’Her. (Myrtaceae). Aust Syst Bot 13:79–148
Brown AG, Eldridge KG, Green JW, Matheson AC (1976) Genetic variation of Eucalyptus obliqua in field trials. New Phytol 77:193–203
Commonwealth of Australia (1992) National forest policy statement: a new focus for Australia’s forests. Canberra, Australia
Currat M, Ruedi M, Petit RJ, Excoffier L (2008) The hidden side of invasions: massive introgression by local genes. Evolution 62:1908–1920
Davis MB (1976) Pleistocene biogeography of temperate deciduous forests. Geosci Man 13:13–26
Dumolin-Lapègue S, Kremer A, Petit RJ (1999) Are chloroplast and mitochondrial DNA variation species independent in oaks? Evolution 53:1406–1413
Ferris C, King RA, Vainola R, Hewitt GM (1998) Chloroplast DNA recognizes three refugial sources of European oaks and suggests independant eastern and western immigrations to Finland. Heredity 80:584–593
Fletcher M, Thomas I (2007) Holocene vegetation and climate change from near Lake Pedder, south-west Tasmania, Australia. J Biogeogr 34:665–677
Flint AW, Fagg P (2007) Mountain Ash in Victoria's State forests, silvicultural reference manual no 1. Department of Sustainability and the Environment, Melbourne
Freeman JS, Jackson HD, Steane DA, McKinnon GE, Dutkowski GW, Potts BM, Vaillancourt RE (2001) Chloroplast DNA phylogeography of Eucalyptus globulus. Aust J Bot 49:585–596
Griffin AR, Burgess I, Wolf L (1988) Patterns of natural and manipulated hybridisation in the genus Eucalyptus L'Hér.: a review. Aust J Bot 36:41–66
Heuertz M, Carnevale S, Fineschi S, Sebastiani F, Hausman JF, Paule L, Vendramin GG (2006) Chloroplast DNA phylogeography of European ashes, Fraxinus sp. (Oleaceae): roles of hybridization and life history traits. Mol Ecol 15:2131–2140
Hewitt GM (1996) Some genetic consequences of ice ages, and their role in divergence and speciation. Biol J Linn Soc 58:247–276
Hoffmann AA, Sgrò CM (2011) Climate change and evolutionary adaptation. Nature 470:479–485
Hughes L (2003) Climate change and Australia: trends, projections and impacts. Austral Ecol 28:423–443
Jackson S, Overpeck J (2000) Responses of plant populations and communities to environmental changes of the late quaternary. Paleobiology 26:194–220
Jackson HD, Steane DA, Potts BM, Vaillancourt RE (1999) Chloroplast DNA evidence for reticulate evolution in Eucalyptus (Myrtaceae). Mol Ecol 8:739–751
Kershaw AP, D'Costa DM, McEwen-Mason J, Wagstaff BE (1991) Palynological evidence for Quaternary vegetation and environments of mainland southeastern Australia. Quat Sci Rev 10:391–404
Kershaw AP, McKenzie GM, Porch N, Roberts RG, Brown J, Heijnis H, Orr ML, Jacobsen G, Newall PR (2007) A high-resolution record of vegetation and climate through the last glacial cycle from Caledonia Fen, southeastern highlands of Australia. J Quat Sci 22:481–500
Kirkpatrick JB, Fowler M (1998) Locating likely glacial forest refugia in Tasmania using palynological and ecological information to test alternative climatic models. Biol Conserv 85:171–182
Ladiges PY, Newnham MR, Humphries CJ (1989) Systematics and biogeography of the Australian “green ash” eucalypts (Monocalyptus). Cladistics 5:345–364
Ladiges PY, Prober SM, Nelson GI (1992) Cladistic and biogeographic analysis of the ‘blue ash’ eucalypts. Cladistics 8:103–124
Ladiges PY, Bayly MJ, Nelson GI (2010) East–west continental vicariance in Eucalyptus subgenus Eucalyptus. In: Williams DM, Knapp S (eds) Beyond cladistics: the branching of a paradigm. University of California Press, Berkeley, pp 267–302
Macphail MK, Colhoun EA (1985) Late glacial vegetation, climates and fire activity in southwest Tasmania. Search 16:43–45
Macphail MK, Jackson W (1978) The Late Pleistocene and Holocene history of the midlands of Tasmania, Australia. Proc R Soc Vic 90:287–300
Maliouchenko O, Palme AE, Buonamici A, Vendramin GG, Lascoux M (2007) Comparative phylogeography and population structure of European Betula species, with particular focus on Betula pendula and Betula pubescens. J Biogeogr 34:1601–1610
McKenzie GM (1997) The late Quaternary vegetation history of the south-central highlands of Victoria, Australia: 1. Sites above 900 m. Aust J Ecol 22:19–36
McKenzie GM (2002) The late Quaternary vegetation history of the south-central highlands of Victoria, Australia: II. Sites below 900 m. Austral Ecol 27:32–54
McKenzie GM, Kershaw AP (1997) A vegetation history and quantitative estimate of Holocene climate from Chapple Vale, in the Otway region of Victoria, Australia. Aust J Bot 45:565–581
McKenzie GM, Kershaw AP (2000) The last glacial cycle from Wyelangta, the Otway region of Victoria, Australia. Palaeogeogr Palaeocol 155:177–193
McKenzie GM, Kershaw AP (2004) Holocene pollen record from cool temperate rainforest, Aire crossing, the Otway region of Victoria, Australia. Rev Palaeobot Palynol 132:281–290
McKinnon GE, Steane DA, Potts BM, Vaillancourt RE (1999) Incongruence between chloroplast and species phylogenies in Eucalyptus subgenus Monocalyptus (Myrtaceae). Am J Bot 86:1038–1046
McKinnon GE, Vaillancourt RE, Jackson HD, Potts BM (2001) Chloroplast sharing in the Tasmanian eucalypts. Evolution 55:703–711
McKinnon GE, Jordan GJ, Vaillancourt RE, Steane DA, Potts BM (2004a) Glacial refugia and reticulate evolution: the case of the Tasmanian eucalypts. Philos Trans R Soc B 359:275–284
McKinnon GE, Vaillancourt RE, Steane DA, Potts BM (2004b) The rare silver gum, Eucalyptus cordata, is leaving its trace in the organellar gene pool Eucalyptus globulus. Mol Ecol 13:3751–3762
Meudt HM, Bayly MJ (2008) Phylogeographic patterns in the Australasian genus Chionohebe (Veronica s.l., Plantaginaceae) based on AFLP and chloroplast DNA sequences. Mol Phylogenet Evol 47:319–338
Muhlfeld CC, Kalinowski ST, McMahon TE, Taper ML, Painter S, Leary RF, Allendorf FW (2009) Hybridization rapidly reduces reproductive success of a native trout in the wild. Biol Lett 5:328–331
Nevill PG, Bossinger G, Ades PK (2010) Phylogeography of the world's tallest angiosperm Eucalyptus regnans: evidence for multiple isolated quaternary refugia. J Biogeogr 37:179–192
Palme AE, Su Q, Palsson S, Lascoux M (2004) Extensive sharing of chloroplast haplotypes among European birches indicates hybridization among Betula pendula, B. pubescens and B. nana. Mol Ecol 13:167–178
Petit RJ, Pineau E, Demesure B, Bacilieri R, Ducousso A, Kremer A (1997) Chloroplast DNA footprints of postglacial recolonization by oaks. Proc Natl Acad Sci U S A 94:9996–10001
Petit RJ, Bodenes C, Ducousso A, Roussel G, Kremer A (2004) Hybridization as a mechanism of invasion in oaks. New Phytol 161:151–164
Pollock LJ, Bayly MJ, Nevill PG, Vesk PA (2013) Chloroplast DNA diversity associated with protected slopes and valleys for hybridizing Eucalyptus species on isolated ranges in south-eastern Australia. J Biogeogr 40:155–167
Pons O, Petit RJ (1996) Measuring and testing genetic differentiation with ordered versus unordered alleles. Genetics 144:1237–1245
Potts BM, Reid JB (1988) Hybridization as a dispersal mechanism. Evolution 42:1245–1255
Remington CL (1968) Suture-zones of hybrid interaction between recently joined biotas. In: Dobzhansky T, Hecht M, Steere W (eds) Evolutionary biology. Plenum, New York, pp 321–428
Sigleo W, Colhoun E (1981) A short pollen diagram from Crown Lagoon in the Midlands of Tasmania. Pap Proc R Soc Tas 115:181–188
Steane DA, Byrne M, Vaillancourt RE, Potts BM (1998) Chloroplast DNA polymorphism signals complex interspecific interactions in Eucalyptus (Myrtaceae). Aust Syst Bot 11:25–40
Steane DA, Jones RC, Vaillancourt RE (2005) A set of chloroplast microsatellite primers for Eucalyptus (Myrtaceae). Mol Ecol Notes 5:538–541
Steane DA, Nicolle D, Sansaloni CP, Petroli CD, Carling J, Kilian A, Myburg AA, Grattapaglia D, Vaillancourt RE (2011) Population genetic analysis and phylogeny reconstruction in Eucalyptus (Myrtaceae) using high-throughput, genome-wide genotyping. Mol Phylogenet Evol 59:206–224
Szpiech ZA, Jakobsson M, Rosenberg NA (2008) ADZE: a rarefaction approach for counting alleles private to combinations of populations. Bioinformatics 24:2498–2504
Tibbits J, McManus L, Spokevicius A, Bossinger G (2006) A rapid method for tissue collection and high throughput genomic DNA isolation from mature trees. Plant Mol Biol Rep 24:1–11
Worth JR, Jordan GJ, McKinnon GE, Vaillancourt RE (2009) The major Australian cool temperate rainforest tree Nothofagus cunninghamii withstood Pleistocene glacial aridity within multiple regions: evidence from the chloroplast. New Phytol 182:519–532
Worth JR, Jordan GJ, Marthick JR, McKinnon GE, Vaillancourt RE (2010) Chloroplast evidence for geographic stasis of the Australian bird-dispersed shrub Tasmannia lanceolata (Winteraceae). Mol Ecol 19:2949–2963
Worth JR, Marthick JR, Jordan GJ, Vaillancourt RE (2011) Low but structured chloroplast diversity in Atherosperma moschatum (Atherospermataceae) suggests bottlenecks in response to the Pleistocene glacials. Ann Bot 108:1247–1256
Wu C, Campbell D (2005) Cytoplasmic and nuclear markers reveal contrasting patterns of spatial genetic structure in a natural Ipomopsis hybrid zone. Mol Ecol 14:781–792
Acknowledgements
We thank Forestry Tasmania, the Department of Sustainability and the Environment Victoria and Vicforests for assistance in sample collection. This work was supported by the Cooperative Research Centre for Forestry and an Australian Postgraduate Award for PN.
Data Archiving Statement
Chloroplast microsatellite data has been submitted to the TreeGenes Database. Accession numbers are: E. delegatensis, TGDR 024; E. obliqua, TGDR 026; E. regnans, TGDR 027. Each accession number is presented with its allelic composition for the five loci used in our study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Y. Tsumura
Rights and permissions
About this article
Cite this article
Nevill, P.G., Després, T., Bayly, M.J. et al. Shared phylogeographic patterns and widespread chloroplast haplotype sharing in Eucalyptus species with different ecological tolerances. Tree Genetics & Genomes 10, 1079–1092 (2014). https://doi.org/10.1007/s11295-014-0744-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11295-014-0744-y