Abstract
Objectives
L-3-[18F]-Fluoro-α-methyl tyrosine (FAMT), an amino acid positron emission tomography (PET) tracer, complements [18F]-fluorodeoxyglucose (FDG) in the diagnosis of malignancies. We compared the predictive ability of FAMT PET versus FDG PET regarding metastatic oral squamous cell carcinoma (OSCC) outcomes for distant metastasis, including lymph node metastasis, and identified the relevant metabolic parameters for each.
Methods
We enrolled 160 patients with OSCC who underwent PET/computed tomography using FDG and FAMT before treatment. Outcomes were assessed using clinicopathological characteristics such as the standardized uptake value (SUVmax, SUVpeak), metabolic tumor volume (MTV), and total lesion glycolysis or total lesion retention. Univariate and multivariate Cox proportional hazards models were used to identify the independent predictors of disease-free survival (DFS) and overall survival (OS) during an average follow-up time of 1401.7 and 1646.0 days, respectively. Areas under the receiver operating characteristic curves were analyzed for the accuracy and predictive value of imaging parameters.
Results
Clinical parameters (excluding age) and PET metabolic parameters were significantly associated with OS. Multivariate analysis showed that an infiltrative growth pattern [p = 0.034, hazard ratio (HR) = 2.30], and the FDG-measured SUVpeak (p = 0.045, HR = 2.45) were independent risk factors for DFS and that lymph node metastasis (p = 0.03, HR = 2.57) and the FAMT-measured MTV (p = 0.004, HR = 3.65) were independent risk factors for OS.
Conclusions
In patients with OSCC, FDG PET predicted DFS, whereas FAMT predicted OS. The two PET tracers, combined with clinical parameters, provide complementary, outcome-related diagnostic information in OSCC.
Similar content being viewed by others
Introduction
Lip and oral cavity cancers have an incidence of 1.2% and represent 1.0% of all-cancer mortality. They were responsible for almost 3,945 deaths among the 409,399 cancer-related deaths in Japan in 2018 [1]. Oral squamous cell carcinoma (OSCC) represents approximately 30% of head and neck cancers and is the most frequent type of such cancers [2]. The use of prognostic parameters such as clinicopathological factors, anatomical location, and TNM stage in such cases still fails to identify those patients in need of intensive versus standard therapy prior to treatment [3].
[18F]-Fluorodeoxyglucose (FDG) is a radiologic analog of glucose through which positron emission tomography (PET) enables the visualization of glucose metabolism; it is useful as a marker of tumor metabolic activity in terms of cell viability and proliferation [4, 5]. Analyses of the metabolic tumor volume (MTV) and total lesion glycolysis (TLG) by 18FDG PET have been used to investigate the clinical and prognostic ability of the technique, as indicated by parameters such as survival and occult metastasis, in patients with OSCC [6].
FDG is the most widely used PET radiotracer for the diagnosis, characterization, and staging of malignant oral cancer because of its high sensitivity [7]. Nevertheless, the tracer lacks specificity because of its inability to identify the tumor as malignant or benign; FDG accumulates in both benign tumors and inflammatory lesions, causing false positives to occur [8]. Accurate assessment of local involvement is important to minimize the extent of surgery, given that surgical treatment usually involves the removal of oral cavity organs or mandibular segmentectomy in patients with oral cancers. Surgery is also hampered by the complexities of the head/neck anatomy, which increase the need for a precise diagnosis of tumor invasion [9]. Therefore, new PET radiotracers with higher specificity are needed for the accurate evaluation of tumor size.
L-[3-18F]-α-Methyl tyrosine (FAMT) is an amino acid tracer that has been developed for PET imaging and that shows better specificity for cancer diagnosis than FDG PET [10]. FAMT is incorporated into cancer cells by L-type amino acid transporter 1 (LAT1), which is only overexpressed in malignant tumors. This overexpression is triggered by cell proliferation and angiogenesis in various human cancers, including brain, lung, prostatic, breast, pancreatic, gastric, urinary tract, and esophageal cancers [11, 12]. Low expression of LAT1 has been reported in adenocarcinoma (29% in pulmonary adenocarcinoma [13], 22% in prostate cancer [14], 43% in breast cancer [15], 52% in pancreatic cancer [16], and 43% in gastric cancer [17]). Conversely, this transporter is highly expressed in squamous cell carcinoma (91% in pulmonary squamous cell carcinoma [13] and 50% in oral cancer [11]). We have reported that the specificity of FAMT PET for malignant tumors is higher than that of FDG PET and that tumor delineation by FAMT PET is also superior to that by FDG PET [11]. However, the ability of FAMT PET to predict disease-free survival (DFS) or overall survival (OS) outcomes in patients with OSCC is unknown. Therefore, this study was designed to compare the utility of FAMT PET-derived and FDG PET-derived metabolic parameters in predicting clinical outcomes in patients with OSCC.
Materials and methods
Study population
We retrospectively evaluated 160 patients who underwent FDG PET and FAMT PET with computed tomography (CT) before treatment at our institution from April 2008 to June 2015; the patient group comprised 96 men and 64 women with a median age of 69.2 years (range 27–93 years). The examined clinical factors were adjusted for age, sex, and the location of the tumor. OSCC was diagnosed based on pathological findings and was confirmed in all patients by a pathologist. The TNM stage was classified using the UICC 2009 TNM cancer staging system (ver. 7) during the data collection. The infiltrative growth pattern (INF) was used as a measured variable in the univariate analysis, and each tumor was classified as INF-a (extensive growth of tumor nests with a well-defined border from surrounding tissue), INF-b (intermediate growth pattern between INF-a and -c), or INF-c (infiltrative growth of tumor nests with an ill-defined border from surrounding tissue) [18]. The data obtained from the medical records were clinical variables, treatment, and follow-up events (lymph node metastasis or recurrence). Informed consent was obtained from all patients for involvement in this study. This study was approved by the institutional review board (IRB number: 2017 − 254).
PET imaging and analysis
PET volumetric parameters were computed from attenuation-corrected PET data using a syngo.via device (SIEMENS Healthcare, Erlangen, Germany) and oncology software package (SIEMENS Healthcare). 18F-FDG or 18F-FAMT was intravenously administered to the patients at a dose of 5.0 MBq/kg (median dose: 18F-FDG, 276.6 MBq and 18F-FAMT, 255.5 MBq) after they had fasted for at least 6 h. PET imaging was performed 68.8 ± 16.5 and 67.6 ± 13.7 min after administering 18F-FDG and 18F-FAMT, respectively. PET volumetric parameters were calculated using 18F-FDG and 18F-FAMT thresholds of 2.5 and 1.4, respectively. These cut-off values were in accordance with those in our previous study, suggesting that the cut-off standardized uptake value (SUV) for patients with OSCC required an exclusive threshold [19]. This cut-off value was then used to calculate the PET parameters, which were derived by computerized-assisted reporting via threshold automated segmentation, to define volumes and then automatically calculate the MTV and average SUV. The resulting SUV (SUVmax, SUVpeak) and MTV were used to calculate the TLG for FDG and total lesion retention (TLR) for FAMT using the formula MTV × SUV mean in both cases [20].
Statistical analysis
Receiver operating characteristic curves were used for each metabolic tumor parameter. Univariate analysis was used to identify clinical, pathological, and PET volumetric factors that predict DFS and OS according to the Kaplan–Meier method and log-rank tests. Multivariate Cox proportional hazards model analysis was performed to determine significant odds ratios for DFS and OS. Time periods were calculated according to the Japan Clinical Cancer Research Organization guidelines. Statistical analyses were performed using SPSS software ver. 23 (IBM Corp., Armonk, NY, USA). In all tests, p < 0.05 was considered significant.
Results
Patient population
The demographic characteristics of the patients are shown in Table 1. The measured variables were age and sex, primary tumor site [most common was tongue (n = 60), followed by mandible (n = 49)], histological differentiation (mild and moderate, n = 144; severe, n = 16), INF and pathological TNM stage, and the treatment provided to patients.
Univariate and multivariate analyses
The univariate analysis results are shown in Table 2. Each parameter was evaluated in relation to DFS and OS for an average follow-up time of 1401.7 and 1646.0 days, respectively. The factors examined in the univariate analysis were age, sex, histological differentiation, pathological parameters (INF, tumor size, regional lymph node metastasis, and presence of distant metastasis), stage, and PET-derived parameters for FDG and FAMT (SUVmax, SUVpeak, MTV, and TLG/TLR). All of the tested parameters were significantly associated with OS regardless of age (p < 0.05).
The FDG PET parameters MTV (g/mL) (≤ 8.51, n = 80; >8.51, n = 80) and TLG (bw × cm3) (≤ 29.8, n = 77; >29.8, n = 83) were not significantly associated with DFS, whereas SUVmax and SUVpeak were significantly associated with DFS (p = 0.010 and 0.005, respectively). The FAMT PET parameters SUVmax (g/mL) (≤ 2.8, n = 75; >2.8, n = 85) and SUVpeak (g/mL) (≤ 2.3, n = 85; >2.3, n = 75) were not significantly associated with DFS; however, MTV and TLR were significantly associated with DFS (p = 0.014 and 0.040, respectively). Of the pathological parameters, histological differentiation, INF, TNM stage, and overall stage were significantly associated with DFS (p < 0.05).
The multivariate analysis incorporated INF, regional lymph node metastasis, SUVpeak on FDG PET, and MTV on FAMT PET (Table 3). The results indicated that INF [hazard ratio (HR) = 2.30, p = 0.034] and SUVpeak (HR = 2.45, p = 0.045) were significantly associated with DFS and that lymph node metastasis (HR = 2.57, p = 0.03) and MTV (HR = 3.65, p = 0.004) were significant and independent risk factors for OS.
Representative cases
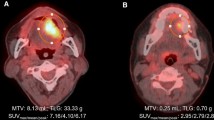
Figures 1 and 2 show representative cases in this study. Figure 1 shows a 51-year-old man with right tongue cancer (T4aN0M0, Stage IVA). 18F-FDG PET/CT scans showed intense accumulation of 18F-FDG (PET parameters: SUVmax, 4.2 g/ml; SUVpeak, 3.3 g/ml; MTV, 3.4 g/ml; TLG, 10.6 bw × cm3) (Fig. 1c); however, 18F-FAMT scans did not show intense accumulation (PET parameters: SUVmax, 1.7 g/ml; SUVpeak, 1.5 g/ml; MTV, 1.2 g/ml; TLR, 1.8 bw × cm3) in this patient (Fig. 1d). The patient outcomes were censored. Figure 2 shows a 63-year-old man with right tongue cancer (T2N2bM0, Stage IVA). 18F-FDG PET/CT scans showed intense accumulation of 18F-FDG (PET parameters: SUVmax, 6.4 g/ml; SUVpeak, 4.4 g/ml; MTV, 5.0 g/ml; TLG, 18.2 bw × cm3) (Fig. 2c), and 18F-FAMT scans also showed intense accumulation (PET parameters: SUVmax, 2.3 g/ml; SUVpeak, 1.8 g/ml; MTV, 6.1 g/ml; TLG, 10.5 bw × cm3) in this patient (Fig. 2d). The patient outcome was local recurrence (160 days postoperatively).
Discussion
We found that FAMT uptake calculated in terms of MTV and lymph node metastasis were independent predictive factors for OS, while SUVpeak from FDG PET was not prognostic for OS. These results suggest that in patients with OSCC, FDG PET-derived SUVpeak is useful for predicting the short-term response because of the high sensitivity of the FDG radiotracer relative to FAMT, whereas FAMT PET is potentially clinically useful for predicting longer-term outcomes (e.g., survival) because of its relatively higher specificity.
FDG has been the gold standard for PET imaging of malignancies. However, detection of lesions with FDG depends on the glycolytic activity of inflammatory tissues that can potentially obscure tumors and increase false positives [18]. Insufficient standardization and reproducibility in determining the therapeutic response may also hamper the use of FDG as a prognostic marker [21]. We previously reported that OSCC lesions were overestimated when using FDG PET volumetric parameters because of localized inflammation [19]. FAMT was developed specifically to solve the issue of false positives in FDG PET [8, 12, 22, 23]. This is the first study to evaluate FAMT PET and its volumetric parameters as predictors of outcomes in OSCC. Volumetric parameters appear to be useful as prognostic markers in patients with OSCC given that parameters such as MTV and TLG reflect the whole tumor burden compared with SUVmax. Volumetric parameters can be used during radiation or chemotherapy to directly visualize the metabolic reaction of the malignancy [24]. Eventually, such parameters might be assessed to derive whole tumor information, including one-point pixel information, which is considered to be a more reliable predictor of DFS and OS than is SUVmax.
Pathological invasion is considered an independent prognostic factor in patients with OSCC. In clinical practice, the clinical and histopathological parameters derived from specimen biopsy and resection are the most common factors in deciding on a treatment strategy and determining the prognosis [25]. Lymph node metastasis and OS in patients with OSCC can also be assessed pathologically.
We hypothesize that FDG will prove to be better at defining localized lesions because of its high sensitivity and will be suitable for determination of DFS. In contrast, FAMT is likely to be more effective in evaluating the behavior of malignant tumors and would be more appropriate for determining OS. DFS itself is a better predictor of short-term therapeutic responses after initial therapy and can thus be a better strategy for predicting primary treatment effectiveness; it would also improve treatment guidance. OS, however, is a better predictor of long-term therapeutic responses. Hence, the combination of FDG PET and FAMT PET will provide superior predictive imaging results in the clinical setting.
This study had several limitations. First, this study was not directly focused on cell biomarkers (e.g., Ki-67 immunohistochemistry or LAT1 expression) that are closely associated with tumor cell proliferation, the grade of malignancy, and poor outcomes. Second, the semiquantitative evaluation in this study was dependent on the software used to derive the data. Thus, the thresholds used for PET volumetric parameter calculation were determined automatically, and accuracy would suffer if the thresholds were not correct. Therefore, a further study using a combination of clinical data and pathologic data is highly recommended. Finally, the images used automated tumor delineation based on fixed thresholds, which may have also reduced the accuracy.
FDG SUVpeak and FAMT MTV are significant predictors of DFS and OS, respectively, in patients with OSCC. Our data suggest that volumetric PET imaging parameters might be able to predict both short- and long-term outcomes and possibly do so as well as clinicopathologic predictors.
References
International Agency for Research on Cancer [Internet]. GLOBOCAN: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2018. Cancer Research, UK [cited 2018 Dec 19]. http://gco.iarc.fr/today/data/factsheets/populations/392-japan-fact-sheets.pdf, https://www.iarc.fr, https://www.uicc.org, https://www.cancerresearchuk.org
Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2013;63:11–30.
Allal AS, Slosman DO, Kebdani T, Allaoua M, Lehmann W, Dulguerov P. Prediction of outcome in head-and-neck cancer patients using the standardized uptake value of 2-[18F]fluoro-2-deoxy-D-glucose. Int J Radiat Oncol Biol Phys. 2004;59:1295–300.
Minn H, Clavo AC, Grenman R, Wahl RL. In vitro comparison of cell proliferation kinetics and uptake of tritiated fluorodeoxyglucose and L-methionine in squamous-cell carcinoma of the head and neck. J Nucl Med. 1995;36:252–8.
Haberkorn U, Strauss LG, Reisser C, Haag D, Dimitrakopoulou A, Ziegler S, et al. Glucose uptake, perfusion, and cell proliferation in head and neck tumors: relation of positron emission tomography to flow cytometry. J Nucl Med. 1991;32:1548–55.
Ryu IS, Kim JS, Roh JL, Cho KJ, Choi SH, Nam SY, et al. Prognostic significance of preoperative metabolic tumour volume and total lesion glycolysis measured by 18F-FDG PET/CT in squamous cell carcinoma of the oral cavity. Eur J Nucl Med Mol Imaging. 2014;4:452.
Seitz O, Chambron-Pinho N, Middendorp M, Sader R, Mack M, Vogl TJ, et al. 18F-Fluorodeoxyglucose-PET/CT to evaluate tumor, nodal disease, and gross tumor volume of oropharyngeal and oral cavity cancer: comparison with MR imaging and validation with surgical specimen. Neuroradiology. 2009;51:677–86.
Kaira K, Oriuchi N, Otani Y, Yanagitani N, Sunaga N, Hisada T, et al. Diagnostic usefulness of fluorine-18-alpha-methyltyrosine positron emission tomography in combination with 18F-fluorodeoxyglucose in sarcoidosis patients. Chest. 2007;131:1019–27.
Pfister DG, Ang KK, Brizel DM, Burtness BA, Cmelak AJ, Colevas AD, et al. Head and neck cancers. J Natl Compr Canc Netw. 2011;9:596–650.
Tomiyoshi K, Amed K, Muhammad S, Higuchi T, Inoue T, Endo K, et al. Synthesis of isomers of 18F-labelled amino acid radiopharmaceutical: position 2- and 3-L-18F-alpha-methyltyrosine using a separation and purification system. Nucl Med Commun. 1997;18:169–75.
Nobusawa A, Kim M, Kaira K, Miyashita G, Negishi A, Oriuchi N, et al. Diagnostic usefulness of 18F-FAMT PET and L-type amino acid transporter 1 (LAT1) expression in oral squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2013;40:1692.
Kaira K, Oriuchi N, Otani Y, Shimizu K, Tanaka S, Imai H, et al. Fluorine-18-alpha-methyltyrosine positron emission tomography for diagnosis and staging of lung cancer: a clinicopathologic study. Clin Cancer Res. 2007;13:6369–78.
Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, et al. Prognostic significance of L-type amino acid transporter 1 expression in resectable stage I-III nonsmall cell lung cancer. Br J Cancer. 2008;98:742–8.
Sakata T, Ferdous G, Tsuruta T, Satoh T, Baba S, Muto T, et al. L-type amino acid transporter 1 as a novel biomarker for high-grade malignancy in prostate cancer. Pathol Int. 2009;59:7–18.
Furuya M, Horiguchi J, Nakajima H, Kanai Y, Oyama T. Correlation of L-type amino acid transporter 1 and CD98 expression with triple negative breast cancer prognosis. Cancer Sci. 2012;103:382–9.
Kaira K, Sunose Y, Arakawa K, Ogawa T, Sunaga N, Shimizu K, et al. Prognostic significance of L-type amino-acid transporter 1 expression in surgically resected pancreatic cancer. Br J Cancer. 2012;107:632–8.
Ichinoe M, Mikami T, Yoshida T, Igawa I, Tsuruta T, Nakada N, et al. High expression of L-type amino-acid transporter 1 (LAT1) in gastric carcinomas: comparison with non-cancerous lesions. Pathol Int. 2011;61:281–9.
Ebisumoto K, Okami K, Ogura G, Sakai A, Sugimoto R, Saito K, et al. The predictive role of infiltrative growth pattern in early pharyngeal cancers. Acta Otolaryngol. 2015;135:1172–7.
Kim M, Achmad A, Higuchi T, Arisaka Y, Yokoo H, Yokoo S, et al. Effects of intratumoral inflammatory process on 18F-FDG uptake: pathologic and comparative study with 18F-fluoro-α-methyltyrosine PET/CT in oral squamous cell carcinoma. J Nucl Med. 2015;56:16–21.
Kim M, Higuchi T, Arisaka Y, Achmad A, Tokue A, Tominaga H, et al. Clinical significance of 18F-a-methyl tyrosine PET/CT for the detection of bone marrow invasion in patients with oral squamous cell carcinoma: comparison with 18F-FDG PET/CT and MRI. Ann Nucl Med. 2013;27:423–30.
Kaira K, Higuchi T, Sunaga N, Arisaka Y, Hisada T, Tominaga H, et al. Usefulness of 18F-α-methyltyrosin PET as therapeutic monitoring of patients with advance lung cancer. Anticancer Res. 2016;36(12):6481–90.
Nishii R, Higashi T, Kagawa S, Kishibe Y, Takahashi M, Yamauchi H, et al. Diagnostic usefulness of an amino acid tracer, α-[N-methyl-11C]methylaminoisobutyric acid (11C- MeAIB), in the PET diagnosis of chest malignancies. Ann Nucl Med. 2013;27:808–21.
Leskinen-Kallio S, Ruotsalainen U, Någren K, Teräs M, Joensuu H. Uptake of carbon-11-methionine and fluorodeoxyglucose in non-Hodgkin’s lymphoma: a PET study. J Nucl Med. 1991;32:1211–8.
Im HJ, Oo S, Jung W, Jang JY, Kim SW, Cheon GJ, et al. Prognostic value of metabolic and volumetric parameters of preoperative FDG-PET/CT in patients with resectable pancreatic cancer. Medicine. 2016;95:e3686.
Carreras-Torras C, Gay-Escoda C. Techniques for early diagnosis of oral squamous cell carcinoma: systematic review. Med Oral Patol Oral Cir Bucal. 2015;20:305–15.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 16J40211.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mai Kim, Tetsuya Higuchi, Takahito Nakajima, Putri Andriana, Hiromi Hirasawa, Azusa Tokue, Jun Kurihara, Satoshi Yokoo, and Yoshito Tsushima declare that they have no conflict of interest.
Human rights statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Informed consent
Informed consent was obtained from all patients for involvement in this study.
Animal rights statement.
This article does not contain any studies with animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kim, M., Higuchi, T., Nakajima, T. et al. 18F-FDG and 18F-FAMT PET-derived metabolic parameters predict outcome of oral squamous cell carcinoma. Oral Radiol 35, 308–314 (2019). https://doi.org/10.1007/s11282-019-00377-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11282-019-00377-2