Abstract
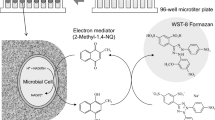
The ethanol extracts of 155 different foodstuffs containing medicinal plants were investigated for their biofilm eradication activities against pathogenic bacteria. A combined method of a colorimetric microbial viability assay based on reduction of a tetrazolium salt (WST-8) and a biofilm formation technique on the 96-pins of a microtiter plate lid was used to screen the biofilm eradication activities of foodstuffs. The ethanol extracts of licorice (Glycyrrhiza glabra) showed potent biofilm eradication activities against Streptococcus mutans, Staphylococcus aureus, and Porphyromonas gingivalis. Among the antimicrobial constituents in licorice, glabridin had the most potent eradication activities against microbial biofilms. The minimum biofilm eradication concentration of glabridin was 25–50 μg/ml. Furthermore, the combination of glabridin with ɛ-poly-l-lysine, a food additive, could result in broad biofilm eradication activities towards a wide variety of bacteria associated with infection, including Escherichia coli and Pseudomonas aeruginosa.

Similar content being viewed by others
References
Antolak H, Kregiel D, Czyzowska A (2015) Adhesion of Asaia bogorensis to glass and polystyrene in the presence of cranberry juice. J Food Protect 78:1186–1190
Antolak H, Czyzowska A, Sakač M, Mišan A, Đuragič O, Kregiel D (2017) Phenolic compounds contained in little-known wild fruits as antiadhesive agents against the beverage-spoiling bacteria Asaia spp. Molecules 22:1256–1274
Bassyouni RH, Kamel Z, Megahid A, Samir E (2012) Antimicrobial potential of licorice: leaves versus roots. Afr J Microbiol Res 6:7485–7493
Bhattacharya M, Wozniak DJ, Stoodley P, Hall-Stoodley L (2015) Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev Anti-Infect Ther 13:1499–1516
Burmølle M, Thomsen TR, Fazli M, Dige I, Christensen L, Homøe P, Tvede M, Nyvad B, Tolker-Nielsen T, Givskov M, Moser C, Kirketerp-Møller K, Johansen HK, Høiby N, Jensen PØ, Sørensen SJ, Bjarnsholt T (2010) Biofilms in chronic infections—a matter of opportunity monospecies biofilms in multispecies infections. FEMS Immunol Med Microbiol 59:324–336
Capita R, Alonso-Calleja C (2013) Antibiotic-resistant bacteria: a challenge for the food industry. Crit Rev Food Sci Nutr 53:11–48
Chatterji M, Shandil R, Manjunatha MR, Solapure S, Ramachandran V, Kumar N, Saralaya R, Panduga V, Reddy J, Prabhakar KR, Sharma S, Sadler C, Cooper CB, Mdluli K, Iyer PS, Narayanan S, Shirude PS (2014) 1,4-Azaindole, a potential drug candidate for treatment of tuberculosis. Antimicrob Agents Chemother 58:5325–5331
Clinical and Laboratory Standards Institute (2012) Methods for dilution microbial susceptibility tests for bacteria that grow aerobically; Approved standard-9th edition M7-A9. Wayne PA USA
Dubois-Brissonnet F, Trotier E, Briandet R (2016) The biofilm lifestyle involves an increase in bacterial membrane saturated fatty acids. Front Microbiol 7:1673
Fukai T, Marumo A, Kaitou K, Kanda T, Terada S, Nomura T (2002) Antimicrobial activity of licorice flavonoids against methicillin-resistant Staphylococcus aureus. Fitoterapia 73:536–539
Gupta VK, Fatima A, Faridi U, Negi AS, Shanker K, Kumar JK, Rahuja N, Luqman S, Sisodia BS, Saikia D, Darokar MP, Khanuja SPS (2008) Antimicrobial potential of Glycyrrhiza glabra roots. J Ethnopharmacol 166:377–380
Hancock V, Dahl M, Vejborg RM, Klemm P (2010) Dietary plant components ellagic acid and tannic acid inhibit Escherichia coli biofilm formation. J Med Microbiol 59:496–498
Hogan S, Zapotoczna M, Stevens NT, Humphreys H, O’Gara JP, O’Neilla E (2016) Eradication of Staphylococcus aureus catheter-related biofilm infections using ML:8 and citrox. Antimicrob Agents Chemother 60:5968–5975
Hongmei Z, Wenyuan Z, Wenyan Z, Anlin Y, Yanlan L, Yan J, Shaosong H, Jianyu S (2014) Inhibitory effects of citral, cinnamaldehyde, and tea polyphenols on mixed biofilm formation by foodborne Staphylococcus aureus and Salmonella enteritidis. J Food Prot 6:872–1042
How KY, Song KP, Chan KG (2016) Porphyromonasgingivalis: an overview of periodontopathic pathogen below the gum line. Front Microbiol 7:53
Koo H, Duarte S, Murata RM, Scott-Anne K, Gregoire S, Watson GE, Singh AP, Vorsa N (2010) Influence of cranberry proanthocyanidins on formation of biofilms by Streptococcus mutans on saliva-coated apatitic surface and on dental caries development in vivo. Caries Res 44:116–126
Luo J, Li Z, Wang J, Weng Q, Chen S, Hu M (2016) Antifungal activity of isoliquiritin and its inhibitory effect against Peronophythora litchi Chen through a membrane damage mechanism. Molecules 21:237–248
Maddocks SE, Lopez MS, Rowlands RS, Coope RA (2012) Manuka honey inhibits the development of Streptococcus pyogenes biofilms and causes reduced expression of two fibronectin binding proteins. Microbiology 158:781–790
Mah TC, O’Toole GA (2001) Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9:134–139
Marsh PD, Zaura E (2017) Dental biofilm: ecological interactions in health and disease. J Clin Periodontol 44:S12–S22
Marsh PD, Head DA, Devine DA (2015) Dental plaque as a biofilm and a microbial community—implications for treatment. J Oral Biosci 57:185–191
Messier C, Grenier D (2011) Effect of licorice compounds licochalcone A, glabridin and glycyrrhizic acid on growth and virulence properties of Candida albicans. Mycoses 54:801–806
Nassar HM, Li M, Gregorya RL (2012) Effect of honey on Streptococcus mutans growth and biofilm formation. Appl Environ Microbiol 78:536–540
Parsons JB, Rock CO (2013) Bacterial lipids: Metabolism and membrane homeostasis. Prog Lipid Res 52:249–276
Pink DA, Hasan FM, Quinn BE, Winterhalter M, Mohan M, Gill TA (2014) Interaction of protamine with gram-negative bacteria membranes: possible alternative mechanisms of internalization in Escherichia coli, Salmonella typhimurium and Pseudomonas aeruginosa. J Pept Sci 20:240–250
Sharma G, Sharma S, Sharma P, Chandola D, Dang S, Gupta S, Gabrani R (2016) Escherichia coli biofilm: development and therapeutic strategies. J Appl Microbiol 121:309–319
Shibata S (2000) A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi 120:849–862
Simmler C, Pauli GF, Chen SN (2013) Phytochemistry and biological properties of glabridin. Fitoterapia 90:160–184
Singh V, Pal A, Darokar MP (2015) A polyphenolic flavonoid glabridin: oxidative stress response in multidrug-resistant Staphylococcus aureus. Free Radic Biol Med 87:48–57
Stojanović-Radić Z, Pejčić M, Stojanović N, Sharifi-Rad J, Stanković N (2016) Potential of Ocimum basilicum L. and Salvia officinalis L. essential oils against biofilms of P. aeruginosa clinical isolates. Cell Mol Biol 62:27–33
Szczepanski S, Lipski A (2014) Essential oils show specific inhibiting effects on bacterial biofilm formation. Food Control 36:224–229
Tada A, Ishizuki K, Sugimoto N, Yoshimatsu K, Kawahara N, Suematsu T, Arifuku K, Fukai T, Tamura Y, Ohtsuki T, Tahara M, Yamazaki T, Akiyama H (2015) Determination of the plant origin of licorice oil extract, a natural food additive, by principal component analysis based on chemical components. Shokuhineiseigaku Zasshi 56:217–227
Tan Z, Shi Y, Xing B, Hou Y, Cui J, Jia S (2019) The antimicrobial effects and mechanism of ε-poly-lysine against Staphylococcus aureus. Bioresour Bioprocess 6:11
Tsukatani T, Higuchi T, Suenaga H, Akao T, Ishiyama M, Ezoe T, Matsumoto K (2009) Colorimetric microbial viability assay based on reduction of water-soluble tetrazolium salts for antimicrobial susceptibility testing and screening of antimicrobial substances. Anal Biochem 393:117–125
Tsukatani T, Kawaguchi T, Suenaga H, Shiga M, Ikegami T (2016) Rapid and simple determination of minimum biofilm eradication concentration by a colorimetric microbial viability assay based on reduction of a water-soluble tetrazolium salt and combined effect of antibiotics against microbial biofilm. J Microbiol Biotechnol Food Sci 6:677–680
Tsukatani T, Sakata F, Kuroda R (2020) A rapid and simple measurement method for biofilm formation inhibitory activity using 96-pin microtiter plate lids. World J Microbiol Biotechnol 36:189
Tsukiyama R, Katsura H, Tokuriki N, Kobayashi M (2002) Antibacterial activity of licochalcone A against spore-forming bacteria. Antimicrob Agents Chemother 46:1226–1230
Wang L, Yang R, Yuan B, Liun Y, Liunn C (2015) The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm Sin B 5:310–315
Wei L, Wu R, Wang C, Wu Z (2017) Effects of ɛ-polylysine on Pseudomonas aeruginosa and Aspergillus fumigatus biofilm in vitro. Med Sci Monit 23:4225–4229
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP25450123.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tsukatani, T., Kuroda, R. & Kawaguchi, T. Screening biofilm eradication activity of ethanol extracts from foodstuffs: potent biofilm eradication activity of glabridin, a major flavonoid from licorice (Glycyrrhiza glabra), alone and in combination with ɛ-poly-l-lysine. World J Microbiol Biotechnol 38, 24 (2022). https://doi.org/10.1007/s11274-021-03206-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-021-03206-z