Abstract
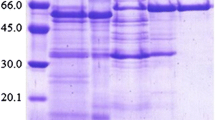
An acid-tolerant α-galactosidase (CVGI) was isolated from the fruiting bodies of Coriolus versicolor with a 229-fold of purification and a specific activity of 398.6 units mg−1. It was purified to electrophoretic homogeneity by ion exchange chromatography and gel filtration chromatography. The purified enzyme gave a single band corresponding to a molecular mass of 40 kDa in SDS-PAGE and gel filtration. The α-galactosidase was identified by MALDI-TOF-MS and its inner peptides were sequenced by ESI-MS/MS. The optimum temperature and pH of the enzyme were determined as 60 °C and 3.0, respectively. The enzyme was very stable at a temperature range of 4–50 °C and at a pH range of 2–5. Among the metal ions tested, Cu2+, Cd2+ and Hg2+ ions have been shown to partially inhibit the activity of α-galactosidase, while the activity of CVGI was completely inactivated by Ag+ ions. N-bromosuccinamide inhibited enzyme activity by 100 %, indicating the importance of tryptophan residue(s) at or near the active site. CVGI had wide substrate specificity (p-nitrophenyl galactoside, melidiose, raffinose and stachyose). After treatment with CVGI, raffinose family oligosaccharide was hydrolyzed effectively to yield galactose and sucrose. The results showed that the general properties of the enzyme offer potential for use of this α-galactosidase in several production processes.




Similar content being viewed by others
References
Anisha GS, Prema P (2008) Reduction of non-digestible oligosaccharides in horse gram and green gram flours using crude a-galactosidase from Streptomyces griseoloalbus. Food Chem 106:1175–1179
Arockiasamy S, Krishnan IP, Anandakrishnan N, Seenivasan S, Sambath A, Venkatasubramani JP (2008) Enhanced production of laccase from Coriolus versicolor NCIM 996 by nutrient optimization using response surface methodology. Appl Biochem Biotechnol 2–3:371–379
Cao YN (2007) Purification and characterization of a novel protease-resistant alpha-galactosidase from Rhizopus sp. F78 ACCC 30795. Enzyme Microb Tech 41:835–841
Cao Y, Wang Y, Luo H, Shi P, Meng K, Zhou Z, Zhang Z, Yao B (2009) Molecular cloning and expression of a novel protease-resistant GH-36 alpha-galactosidase from Rhizopus sp. F78 ACCC 30795. J Microbiol Biotechnol 11:1295–1300
Celem EB, Bolle SS, Onal S (2009) Efficient and rapid purification of lentil alpha-galactosidase by affinity precipitation with alginate. Indian J Biochem Biophys 5:366–370
Cui J, Chisti Y (2003) Polysaccharopeptides of Coriolus versicolor: physiological activity, uses, and production. Biotechnol Adv 2:109–122
Dhananjay SK, Mulimani VH (2009) Three-phase partitioning of alpha-galactosidase from fermented media of Aspergillus oryzae and comparison with conventional purification techniques. J Ind Microbiol Biotechnol 1:123–128
Ferreira JG, Reis AP, Guimaraes VM, Falkoski DL, Fialho LS, de Rezende ST (2011) Purification and characterization of Aspergillus terreus alpha-galactosidases and their use for hydrolysis of soymilk oligosaccharides. Appl Biochem Biotechnol 7:1111–1125
Ghazi S, Rooke JA, Galbraith H (2003) Improvement of the nutritive value of soybean meal by protease and alpha-galactosidase treatment in broiler cockerels and broiler chicks. Br Poult Sci 3:410–418
Goulas T, Goulas A, Tzortzis G, Gibson GR (2009) A novel alpha-galactosidase from Bifidobacterium bifidum with transgalactosylating properties: gene molecular cloning and heterologous expression. Appl Microbiol Biotechnol 3:471–477
Katrolia P, Jia H, Yan Q, Song S, Jiang Z, Xu H (2012) Characterization of a protease-resistant alpha-galactosidase from the thermophilic fungus Rhizomucor miehei and its application in removal of raffinose family oligosaccharides. Bioresour Technol 110:578–586
Kayastha NSAM (2012) A novel application of Cicer α-galactosidase in reduction of raffinose family oligosaccharides in soybean flour. J Plant Biochem Biotechnol. doi:10.1007/s13562-012-0173-7
Kobayashi H, Suzuki H (1972) Studies on the decomposition of raffinose by α-galactosidase of mood. J fermet technol 50:625–632
Kotiguda G, Kapnoor SS, Kulkarni D, Mulimani VH (2007) Degradation of raffinose oligosaccharides in soymilk by immobilized alpha-galactosidase of Aspergillus oryzae. J Microbiol Biotechnol 9:1430–1436
Mi SJ, Bai YG, Meng K, Wang YR, Yao B, Shi XY, Huang HQ, Zhang YH, Shi PJ (2007) Purification and characterization of a novel alpha-galactosidase from penicillium sp. F63 CGMCC1669. Wei Sheng Wu Xue Bao 1:156–160
Miller GL (1957) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–429
Pastore GM, Park YM (1957) Screening of high galactosidase producing fungi and characterizing the hydrolysis properties of a selected strain. J Food Sci 44:1577–1581
Ramalingam SaraswathyN, Sadasivam S, Subha K, Poorani N (2007) Purification and properties of alpha-galactosidase from white-rot fungus Pleurotus florida. Indian J Biochem Biophys 2:76–81
Saad RR, Fawzi EM (2012) Purification and characterization of a thermostable alpha-galactosidase from Thielavia terrestris NRRL 8126 in solid state fermentation. Acta Biol Hung 1:138–150
Shankar SK, Dhananjay SK, Mulimani VH (2009) Purification and characterization of thermostable alpha-galactosidase from Aspergillus terreus (GR). Appl Biochem Biotechnol 2:275–285
Singh N, Kayastha AM (2012) Purification and characterization of alpha-galactosidase from white chickpea (Cicer arietinum). J Agric Food Chem 12:3253–3259
Sripuan T, Aoki K, Yamamoto K, Tongkao D, Kumagai H (2003) Purification and characterization of thermostable alpha-galactosidase from Ganoderma lucidum. Biosci Biotechnol Biochem 7:1485–1491
Viana PA, de Rezende ST, Marques VM, Trevizano LM, Passos FM, Oliveira MG, Bemquerer MP, Oliveira JS, Guimaraes VM (2006) Extracellular alpha-galactosidase from Debaryomyces hansenii UFV-1 and its use in the hydrolysis of raffinose oligosaccharides. J Agric Food Chem 6:2385–2391
Wan JM, Sit WH, Louie JC (2008) Polysaccharopeptide enhances the anticancer activity of doxorubicin and etoposide on human breast cancer cells ZR-75-30. Int J Oncol 3:689–699
Wu ZX, Pang SF, Chen XX, Yu YM, Zhou JM, Chen X, Pang LJ (2012) Effect of Coriolus versicolor polysaccharides on the hematological and biochemical parameters and protection against Aeromonas hydrophila in allogynogenetic crucian carp (Carassius auratus gibelio). Fish Physiol Biochem. doi:10.1007/s10695-012-9689-y
Yeung JH, Or PM (2012) Polysaccharide peptides from Coriolus versicolor competitively inhibit model cytochrome P450 enzyme probe substrates metabolism in human liver microsomes. Phytomedicine 5:457–463
Zeilinger S, Kristufek D, Arisan-Atac I, Hodits R, Kubicek CP (1993) Conditions of formation, purification, and characterization of an alpha-galactosidase of Trichoderma reesei RUT C-30. Appl Environ Microbiol 5:1347–1353
Zhang GQ, Zhang QP, Sun Y, Tian YP, Zhou ND (2012) Purification of a novel pepsin inhibitor from Coriolus versicolor and its biochemical properties. J Food Sci 3:C293–C297
Acknowledgments
This work was supported by National Grants of China (2010CB732202).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Du, F., Liu, Q., Wang, H. et al. Purification an α-galactosidase from Coriolus versicolor with acid-resistant and good degradation ability on raffinose family oligosaccharides. World J Microbiol Biotechnol 30, 1261–1267 (2014). https://doi.org/10.1007/s11274-013-1549-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1549-0