Abstract
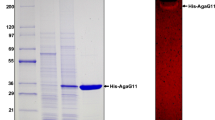
A β-agarase gene hz2 with 2,868 bp was cloned from the marine agarolytic bacterium Agarivorans sp. HZ105. It encoded a mature agarase HZ2 of 102,393 Da (920 amino acids). Based on the amino acid sequence similarity, agarase HZ2 was assigned to the glycoside hydrolase family 50. The β-agarase shared a gene sequence identity of 98.6% with the reported but much less characterized β-agarase agaB from Vibrio sp. JT0107. Its recombinant agarase rHZ2 was produced in E. coli cells and purified to homogeneity. The agarase rHZ2 degraded agarose and neoagarooligosaccharides with degrees of polymerization above four, to yield neoagarotetraose as the dominant product, which was different from β-agarase agaB of Vibrio sp. JT0107. The agarose hydrolysis pattern suggested that rHZ2 was an endo-type β-agarase. Beta-mercaptoethanol (90 mM) and dithiothreitol (9 mM) increased the agarase activity of rHZ2 by 72.9% and 17.3% respectively, while SDS (9 mM) inhibited the activity completely. The agarase activity was independent of Na+, K+, Mg2+ and Ca2+. The maximal enzyme activity was observed at 40°C and pH 7. The kinetic parameters K m, V max, K cat, and K cat/K m values toward agarose of agarase rHZ2 were 5.9 mg ml−1, 235 U mg−1, 401 s−1 and 6.8 × 105 M−1 s−1, respectively. Agarase rHZ2 could have a potential application in the production of bioactive neoagarotetraose.




Similar content being viewed by others
References
Allouch J, Jam M, Helbert W, Barbeyron T, Kloareg B, Henrissat B, Czjzek M (2003) The three-dimensional structures of two β-agarases. J Biol Chem 278:47171–47180
Du Z, Wang J, Yang L, Chen G (2011) Identification of a marine agarolytic bacterium Agarivorans albus QM38 and cloning and sequencing its beta-agarase genes. Acta Oceanol Sin 30:118–124
Fu XT, Kim SM (2010) Agarase: review of major sources, categories, purification method, enzyme characteristics and applications. Mar Drugs 8:200–218
Fu XT, Lin H, Kim SM (2008) Purification and characterization of a novel beta-agarase, AgaA34, from Agarivorans albus YKW-34. Appl Microbiol Biotechnol 78:265–273
Fu XT, Pan C-H, Lin H, Kim SM (2009) Gene cloning, expression, and characterization of a β-agarase, AgaB34, from Agarivorans albus YKW-34. J Microbiol Biotechnol 19:257–264
Hu B, Gong Q, Wang Y, Ma Y, Li J, Yu W (2006) Prebiotic effects of neoagaro-oligosaccharides prepared by enzymatic hydrolysis of agarose. Anaerobe 12:260–266
Hu Z, Lin BK, Xu Y, Zhong MQ, Liu GM (2009) Production of agarase from a marine agarolytic bacterium Agarivorans sp. HZ105. J Appl Microbiol 106:181–190
Jam M, Flament D, Allouch J, Potin P, Thion L, Kloareg B, Czjzek M, Helbert W, Michel G, Barbeyron T (2005) The endo-beta-agarases AgaA and AgaB from the marine bacterium Zobellia galactanivorans: two paralogue enzymes with different molecular organizations and catalytic behaviours. Biochem J 385:703–713
Jang MK, Lee DG, Kim NY, Yu KH, Jang HJ, Lee SW, Jang HJ, Lee Y, Lee S (2009) Purification and characterization of neoagarotetraose from hydrolyzed agar. J Microbiol Biotechnol 19:1197–1200
Kim HT, Lee S, Lee D, Kim HS, Bang WG, Kim KH, Choi IG (2010) Overexpression and molecular characterization of Aga50D from Saccharophagus degradans 2–40: an exo-type β-agarase producing neo- agarobiose. Appl Microbiol Biotechnol 86:227–234
Kobayashi R, Takisada M, Suzuki T, Kirimura K, Usami S (1997) Neoagarobiose as a novel moisturizer with whitening effect. Biosci Biotechnol Biochem 61:162–163
Lee DG, Park GT, Kim NY, Lee EJ, Jang MK, Shin YG, Park GS, Kim TM, Lee JH, Lee JH, Kim SJ, Lee SH (2006) Cloning, expression, and characterization of a glycoside hydrolase family 50 β-agarase from a marine Agarivorans isolate. Biotechnol Lett 28:1925–1932
Lee DG, Jang MK, Lee OH, Kim NY, Ju SA, Lee SH (2008) Over-production of a glycoside hydrolase family 50 β-agarase from Agarivorans sp. JA-1 in Bacillus subtilis and the whitening effect of its product. Biotechnol Lett 30:911–918
Long M, Yu Z, Xu X (2010) A Novel β-Agarase with high pH stability from marine Agarivorans sp. LQ48. Mar Biotechnol 12:62–69
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Nikapitiya C, Oh C, Lee Y, Lee S, Whang I, Lee J (2010) Characterization of a glycoside hydrolase family 50 thermostable beta agarase AgrA from marine bacteria Agarivorans sp. AG17. Fish Aquat Sci 13:36–48
Ohta Y, Hatada Y, Nogi Y, Li Z, Ito S, Horikoshi K (2004) Cloning, expression, and characterization of a glycoside hydrolase family 86 -agarase from a deep-sea Microbulbifer-like isolate. Appl Microbiol Biotechnol 66:266–275
Ohta Y, Hatada Y, Ito S, Horikoshi K (2005) High-level expression of a neoagarobiose-producing β-agarase gene from Agarivorans sp. JAMB-A11 in Bacillus subtilis and enzymic properties of the recombinant enzyme. Biotechnol Appl Biochem 41:183–191
Potin P, Richard C, Rochas C, Kloareg B (1993) Purification and characterization of the α-agarase from Atteromonas agarlyticus (Cataldi) comb.nov., strain GJ1B. Eur J Biochem 214:599–607
Sugano Y, Matsumoto T, Kodama H, Noma M (1993) Cloning and sequencing of agaA, a unique agarase 0107 gene from a marine bacterium, Vibrio sp. strain JT0107. Appl Environ Microbiol 59:3750–3756
Sugano Y, Matsumoto T, Noma M (1994) Sequence analysis of the agaB gene encoding a new beta-agarase from Vibrio sp. strain JT0107. Biochim Biophys Acta 1218:105–108
Wang J, Jiang X, Mou H, Guan H (2004) Anti-oxidation of agar oligosaccharides produced by agarase from a marine bacterium. J Appl Phycol 16:333–340
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 41076106), Guangdong Natural Science Foundation (No. S2011030005257), the Science & Technology Project of Guangdong Province (No. 2009B030803051) and the Key Science and Technology Innovation Project for University by the Department of Education of Guangdong Province (No.CXZD1124).
Author information
Authors and Affiliations
Corresponding author
Additional information
Bokun Lin and Guoyong Lu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lin, B., Lu, G., Zheng, Y. et al. Gene cloning, expression and characterization of a neoagarotetraose-producing β-agarase from the marine bacterium Agarivorans sp. HZ105. World J Microbiol Biotechnol 28, 1691–1697 (2012). https://doi.org/10.1007/s11274-011-0977-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-011-0977-y