Abstract
Bacteria of the Bacillus species have been reported as an important microorganism in fermented soybean products. In the present study, thirty Bacillus isolates were screened from Meju, a Korean soybean fermentation starter. The comparative analysis of 16S rDNA sequences, 16S-23S internal transcribed spacer sequences, phenotypic, and biochemical characterizations revealed three phylogenetically distinct groups namely Bacillus atrophaeus, Bacillus polyfermenticus and Bacillus subtilis. The isolates were assayed for poly-γ-glutamate production and fibrinolytic activity. Among the isolates, B. polyfermenticus exhibited maximum poly-γ-glutamate production and fibrinolytic activity. Moreover, the soybean products fermented by B. polyfermenticus have increased the time taken for coagulation and hemorrhage in mice. The results of the present study clearly indicate the functional role of B. polyfermenticus in fermented soybean products.
Similar content being viewed by others
Introduction
A variety of fermented foods derived from soybean occupies major portion of the diet in Korea, Japan, Thailand and other Southeast Asian countries. These foods are generally prepared by two different methods, a non-salted and semidry fermentation, and the other is salt and water containing fermentation (Reddy et al. 1986). However, the fermentation process, incubation period, temperature and raw materials used for the fermentation are different among the countries.
Cheonggukjang, is a traditional Korean fermented soybean food consumed by majority of the peoples in Korea. The preparation of Cheonggukjang is very similar to that of Natto, a traditional fermented soybean food of Japan. In addition to Cheonggukjang, Kochujang and Doenjang are the other common fermented soybean foods in Korea. The raw material (fermentation starter) used for the preparation of these Kochujang and Doenjang are called Meju, which is different from Koji a Japanese soybean fermentation starter. Koji is made from cooked whole soybean and wheat derived materials, whereas Meju is made from cooked, chopped and molded soybeans. The preparation process of the Meju is also different from Koji. In brief, the soybean lump is dried and fermented for 15–30 days under outdoor conditions in winter seasons to make Meju. On the contrary, Koji is made by inoculating the pure cultures of Aspergillus oryzae or Aspergillus sojae and incubated at 25–30°C for 3 days (Kim et al. 2002). Hence the microbiological characteristics of Meju are also different from Koji.
Fermented soybean foods have several functional properties like antioxidant, antimutagenesis, immuno-modulatory, antithrombosis, and fibrinolytic activity (Cho et al. 2003; Candela and Fouet 2006). Likewise, Korean fermented soybean foods also have similar functional activities. Interestingly, recent studies have reported that the Korean fermented soybean foods have high functional activities than Japanese Natto (Cheigh et al. 1993; Kim et al. 1996, 1998, 2002). Increased functional activity is probably due to the differences in microbiological characteristics of Meju. Therefore, the present study is aimed at isolation and characterization of the functional bacterial strains from Meju in order to improve the quality of the Korean fermented soybean food, and also to use the strains for commercial applications.
Materials and methods
Isolation and identification of bacteria from meju
The lump of Meju was purchased from several traditional small companies located in Sunchang Kochujang Complex (Chungchang, Chonbuk, Korea); Sunchang-Gol Kochujang, Wang-Sil Kochujang, Moon-Jung-Hee Kochujang, and Hyung-Juk-Won, and carefully transported to the laboratory. The bacteria were isolated by serially diluting 1 g of lump in sterile distilled water and 0.1 ml of the appropriate dilution was plated by spread plate technique on LB agar plates. Later, the plates were incubated at 37°C for 24 h and observed for the appearance of bacterial colonies. Morphologically distinct colonies were purified and stored at 4°C for further analysis. Preliminary characterization of the isolates was based on Gram reaction and biochemical tests (API 50CHB). Further identification of the isolates was based on the 16S rDNA and 16S-23S ITS region sequence analysis.
To extract the DNA, the cells were harvested from 10 ml of overnight culture and the pellets were lysed in 1 ml lysis buffer (25% sucrose, 20 mM EDTA, 50 mM Tris–HCl and 5 mg/ml of lysozyme) (Kamala-Kannan et al. 2006). Chromosomal DNA was extracted according to standard procedure of Maniatis et al. (1989). The 16S rDNA was amplified using polymerase chain reaction (PCR) with the universal primers 27f and 1492r (Reysenbach et al. 1992). The PCR was done in a thermocycler (MJ Research, Waltham, USA) using a thermal cyclic condition at 94°C (5 min) followed by 35 cycles at 94°C (1 min), 49°C (2 min) and 72°C (2 min) with a final extension temperature at 72°C for 7 min.
The 5′ 16S-23S ITS region was amplified using PCR with the primers ITS-B1 (5′-GAA GTC GTA ACA AGG-3′) and ITS-B2 (5′-CGG GTA TAT TTG ATA TGC-3′). The PCR was performed under the conditions of 95°C (5 min) followed by 25 cycles at 95°C (30 s), 54°C (30 s) and 72°C (1 min) with a final extension temperature at 72°C for 7 min. The PCR fragments were purified using PCR extraction kit (Qiagen, Valencia, CA, USA) according to the manufacturer protocol. Purified amplicons were cloned in pDrive vectors (Qiagen, Valencia, CA, USA) and transformed into Escherichia coli JM109 cells according to manufacturer’s recommendation. Recombinant plasmids were isolated by modified alkali method and the sizes of the DNA inserts were verified electrophoretically. Recombinants were further confirmed by restriction digestion and sequenced in both forward and reverse direction by using an automated sequencer ABI PRISM (Model 3700, Foster City, CA, USA). The sequences were compared using the BLAST program for identification of the isolates.
Determination of proteolytic activity
Proteolytic activity of the isolates was determined using skim milk agar. The log phase culture of the isolates were spotted on the surface of the skim milk agar plates and incubated at 37°C for 18 h. The development of a clear zone at the inoculation site was considered to be an index on proteolytic activity (Chantawannakul et al. 2002).
Fermentation of soybeans with isolates
Soybeans (approximately 40 g) were washed and soaked in water for 18 h at room temperature. After decanting the water, soaked beans were transferred to the capped bottles (7 cm × 7 cm × 10 cm), autoclaved at 105°C for 20 min, then again autoclaved at 121°C for 20 min, and were cooled to 80°C. Pure cultures of B. atrophaeus SCN1, B. polyfermenticus SCN11 and B. subtilis SCN101 (106 cells/ml) were inoculated individually, and incubated at 35°C for 48 h.
Preparation of pure PGA
The fermented soybeans were mixed with water and vortexed for 10 min. The PGA was separated and purified from the fermented soybeans according to the method of Goto and Kunioka (1992).
Fibrinolytic activity
Approximately 5 g of the fermented soybeans were freeze dried and grinded in sterile mortar and pestle. One gram of the powder was dissolved in 6 ml of the sterile water and fibrinolytic activity of the soup was determined according to Astrup and Mullertz (1952).
Determination of hemorrhage and coagulation time in mouse
Twenty-seven healthy mice were selected for hemorrhage and coagulation studies. Animals were housed in well-ventilated cages at room temperature with a 12 h light/dark cycle. Mice were randomly divided into three groups, with (n = 9) in each group and treated as follows, Group-A (control) was treated with normal mouse chow. Group-B was treated with B. polyfermenticus fermented soybean foods once a day, whereas Group-C was treated with B. atrophaeus fermented soybean foods. Three mice were selected randomly from each group at regular intervals (1st, 2nd and 4th weeks) and assayed for hemorrhage and coagulation time.
The time taken for hemorrhage was determined by the modified tail cutting method of Yang et al. (2002). In brief, the mice tail was transected about 1 cm from the tip using a disposable surgical blade. The time taken for hemorrhage was calculated from the moment of transaction until the bleeding was completely stopped. The blood was wiped with sterile filter paper at every 20 s intervals during bleeding.
The coagulation time of the blood was detected by slide method (Yang et al. 2002). In brief, the mice tail was transected about 1 cm from the tip using a sterile surgical blade. The first drop of the blood was wiped with sterile filter paper and the second drop was placed on the clean glass slide. A sterile needle was placed at the center of the drop and stirred at an interval of 30 s. The coagulation time was calculated from the moment the blood was placed on the slide until a fibrin silk was obtained. Statistical analyses were done with the Statistical Package for Social Sciences (SPSS, version 12).
Results and discussion
Isolation and Identification of the isolates
Thirty morphologically distinct bacterial colonies were observed in the LB agar plates. Gram staining (Gram positive rods) and phenotypic characterizations such as the presence endospores indicate that the isolates were belonging to Bacillus genus (Table 1). To further validate the identification, part of the 16S rDNA was amplified using PCR. PCR amplification of the targeted 16S rDNA resulted in the predicted 1.55 kbp amplicon in all the thirty isolates. The PCR amplified products were sequenced and compared with the available 16S rDNA sequences in NCBI database. Based on the partial sequences comparison by BLAST, the isolates were identified as B. subtilis. The results are consistent with previous works reporting the presence of Bacillus species in Meju and other fermented soybean foods (Kim et al. 1996; Cho et al. 2003; Jung and Chang 2009; Kwon et al. 2009). This could be due to the ability of the Bacillus species to grow at wide pH range (pH 5.5–8.5) and in addition, the production of several proteolytic enzymes and biologically active compounds (PGA) enhance the growth of Bacillus species in fermented foods. Furthermore, the initial boiling of the soybeans in preparation has destroyed the non-Bacillus species and other microorganisms present in the soybeans.
Although the isolates were identified as B. subtilis, results of phenotypic characters (lactose fermentation) and colony morphology in LB agar clearly indicates the presence of more than one type of isolate. Moreover, recent studies have reported that the highly conserved 16S rDNA cannot discriminate among closely related Bacillus strains. The 16S-23S ITS region has much greater variability in sequence and has been successfully used for the differentiation of closely related Bacillus strains (Gurtler and Stanisich 1996; Xu and Cote 2003). Thus to classify the closely related Bacillus species, 16S-23S ITS region of the isolates was amplified using PCR.
PCR amplification of the targeted ITS region resulted in the predicted 450 bp amplicons in all the thirty isolates. The amplicons were sequenced and compared with the available ITS sequences in NCBI database. Based on the sequence comparison, the isolates were classified as B. atrophaeus, B. polyfermenticus, and B. subtilis (Fig. 1). Ten B. atrophaeus strains were designated as SCN1 to SCN10, twelve B. polyfermenticus strains were designated as SCN11 to SCN22, and eight B. subtilis strains were designated as SCN101 to SCN108. Our findings as well as the previous works by Gurtler and Stanisich (1996) and Xu and Cote (2003) provide evidence that the ITS sequences have successfully differentiated the closely related Bacillus species.
DNA sequence comparison of 16S-23S ITS regions isolated from B. atrophaeus (SCN1and SCN2), B. polyfermenticus (SCN11 and SCN12), and B. subtilis (SCN101 and SCN102). Identical bases in neighboring sequence are highlighted in white on the black background. The dashes indicate gaps in the alignments. The arrows above sequences cover the 16S and 23S ribosomal DNA, respectively
Probiotics has emerged as important dietary supplement in recent days and used as a growth promoters, lactose intolerance, antitumour, and anticholestrolaemic effects (Suvarna and Boby 2005). The survival and activity of probiotic organisms are widely studied in both in vitro and in vivo conditions. Nowadays most of these probiotic products are prepared from single bacterial strain or consortium of bacteria isolated from different environments. Among bacteria, spore forming Bacillus sp. are more popular in probiotic preparation (Hong et al. 2005). Bonafide Bacillus sp. includes B. subtilis, B. cereus, B. licheniformis, B. pumilus, B. clausii and B. coagulans. Inooka et al. (1986) reported the probiotic effect of the B. subtilis isolated from soybean fermented food Natto. Lee et al. (2001) reported the probiotic effect of B. polyfermenticus SCD isolated from ‘Bispan’, a probiotic product of South Korea and Japan. Similarly Min et al. (2009) reported the probiotic effect of B. polyfermenticus KJS-2 isolated from ‘Bispan’, a probiotic product of South Korea. Hence, single isolate from each species (B. atrophaeus SCN1, B. polyfermenticus SCN11 and B. subtilis SCN101) was randomly selected and characterized for their functional roles.
Proteolytic activity
Biologically active peptides are the important constituent in fermented soybean foods. These peptides are inactive within the sequence of the parent protein and can be activated by enzymatic hydrolysis. Therefore, a variety of proteases enzyme are required to improve the quality of fermented soybean foods. Though the Bacillus species are reported to produce a variety of intracellular and extra cellular protease enzymes, pronounced differences in proteolytic activity are observed among Bacillus species (Ouoba et al. 2003). Hence the isolates were screened for proteolytic activity in skim milk agar. Presence of clear zones around the colonies in skim milk agar plates indicated the proteolytic activity of the isolates. Interestingly, all the isolates exhibited moderate to high level of proteolytic activity; thus we have fermented the soybeans with B. atrophaeus SCN1, B. polyfermenticus SCN11 and B. subtilis SCN101 individually and analyzed for PGA and fibrinolytic activity.
PGA production
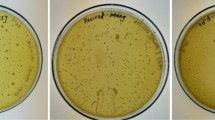
Poly-g-Glutamate (PGA), a polymer of glutamate with a γ-peptide linkage, has several biochemical properties, which facilitate its role differently in food, pharmaceutical and cosmetic industries (Candela and Fouet 2006). Presence of PGA in soybean fermented foods has been reported in several studies (Tanimoto et al. 2001; Candela and Fouet 2006). Nagai et al. (1997) reported that the major slimy material in Natto is PGA. Similarly, the slimy materials were observed in soybeans fermented with B. polyfermenticus SCN11 and B. subtilis SCN101 which indicates the PGA production by isolates. Interestingly, marked difference in the viscosity of slimy material was observed between B. polyfermenticus SCN11 and B. subtilis SCN101 fermented soybeans. Thus, PGA was purified from the fermented soybeans and the molecular size of the compound was measured electrophoretically. The size of B. polyfermenticus SCN11 PGA showing low viscosity was diverse from 90 to 200 kDa, whereas the size of B. subtilis SCN101 PGA showing higher viscosity was higher than 200 kDa. The results are in agreement with the previous report that the difference in the size of PGA varies with organism used/present (Candela and Fouet 2006). However, the slimy material was not observed in B. atrophaeus SCN1 fermented soybeans (Fig. 2).
Fibrinolytic activity
Thrombosis, a cardiovascular disorder reported for a leading cause of death in humans. The improper formation of fibrin (blood clot) in blood vessels is responsible for the thrombosis. Several chemical compounds and fibrinolytic enzymes are identified and successfully used for the treatment (Turpie et al. 2002). Fascinatingly, some studies have reported that the oral administration of fibrinolytic enzymes effectively controlled the thrombosis (Puska et al. 2002; Nestel 2002). Sumi et al. (1990) reported that the oral administration of the fibrinolytic enzyme extracted from Japanese Natto can enhance fibrinolysis in dogs with experimentally induced thrombosis. Hence, the fibrinolytic activity of the fermented soybeans was determined by standard fibrin plate technique. The soybeans fermented with B. polyfermenticus SCN11 exhibited high fibrinolytic activity (0.53 plasmin units) followed by B. atrophaeus SCN1 (0.43 plasmin units) and B. subtilis SCN101 (0.05 plasmin units) (Fig. 3). The results are consistent with the previous study reporting the high variation in fibrinolytic activity among Bacillus species (Kim et al. 1996). Kim et al. (1996) also reported the high fibrinolytic activity of Bacillus sp. isolated from Cheonggukjang. The protease gene in isolated B. polyfermenticus SCN11 was partially amplified by PCR using the primers derived from the consensus sequences of the serine protease. The comparison of the nucleotide sequences indicate that the gene is closely related (90%) with nattokinase gene, a fibrinolytic enzyme encoding gene in B. subtilis natto (data not shown).
Fibrinolytic activities on fibrin plate using the soluble extracts of fermented soybean products as described in materials and methods. 1 B. polymenticus SCN11, 2 B. atrophaeus SCN1, 3 B. subtilis SCN101
Animal model studies
Soybeans fermented by B. polyfermenticus and B. atrophaeus showed high fibrinolytic activity than B. subtilis. Observing the high fibrinolytic activity in in vitro we intend to check the same under in vivo conditions. Hence animal model studies have been performed to evaluate the activity of the enzyme under in vivo conditions. Intrestingly, an increase in hemorrhage and coagulation time was observed in animals which were fed with B. polyfermenticus and B. atrophaeus fermented soybeans (Table 2). The hemorrhage and coagulation time increased according to the incubation period. During the first 2 weeks of incubation a minor increase in the hemorrhage and coagulation time was noticed. Remarkably more than twofold increase in the hemorrhage and coagulation time was noticed in the animals which were fed with 4 weeks. The results are consistent with the previous studies reporting that the regular uptake of soybean fermented foods effectively controlled the thrombosis and other disorders in human beings (Sumi et al. 1990).
Conclusion
The results of the present study clearly indicate that the three bacterial strains namely B. subtilis, B. atrophaeus, and B. polyfermenticus play an important role in the fermentation of Meju. The PGA and protease enzyme produced by the B. polyfermenticus was entirely different from the B. subtilis, a main fermentative microorganism in Natto and other soybean foods. The presence of more than one type of PGA and protease enzymes could be responsible for the increased functional activity of Cheonggukjang when compared with Natto.
References
Astrup T, Mullertz S (1952) The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys 40:346–351
Candela T, Fouet A (2006) Poly-gamma-glutamate in bacteria. Mol Microbiol 60:1091–1098
Chantawannakul P, Oncharoen A, Klanbut K, Chukeatirote E, Lumyong S (2002) Characterization of proteases of Bacillus subtilis strain 38 isolated from traditionally fermented soybean in northern Thailand. Sci Asia 28:241–245
Cheigh HS, Lee JS, Lee CY (1993) Antioxidative characteristics of melanoidin related products fractionated from fermented soybean sauce. J Korean Soc Food Nutr 22:570–575
Cho SJ, Oh SH, Pridmore RD, Juillerat MA, Lee CH (2003) Purification and characterization of proteases from Bacillus amyloliquefaciens isolated from traditional soybean fermentation starter. J Agric Food Chem 51:7664–7670
Goto A, Kunioka M (1992) Biosynthesis and hydrolysis of poly (γ- glutamic acid) from Bacillus subtilis IFO3335. Biosci Biotechnol Biochem 56:1031–1035
Gurtler V, Stanisich VA (1996) New approaches to typing and identification of bacteria using the 16S–23S rDNA spacer region. Microbiology 142:3–16
Hong HA, Duc LH, Cutting SM (2005) The use of bacterial spore formers as probiotics. FEMS Microbiol Rev 29:813–835
Inooka S, Uehara S, Kimura M (1986) The effect of Bacillus natto on the T and B lymphocytes from spleens of feeding chickens. Poult Sci 65:1217–1219
Jung JH, Chang HC (2009) Antifungal activity of Bacillus polyfermenticus CJ6 isolated from Meju. J Korean Soc Food Sci Nutr 38:509–516
Kamala-Kannan S, Mahadevan S, Krishnamoorthy R (2006) Characterization of mercury reducing Bacillus cereus strain isolated from Pulicat Lake sediments, south east of India. Arch Microbiol 185:202–211
Kim WK, Choi KH, Kim YT, Park HH, Choi JY, Lee YS, Oh HI, Kwon IB, Lee SY (1996) Purification and characterization of fibrinolytic enzyme produced from Bacillus sp strain CK 11–4 screened from Chungkook-jang. Appl Environ Microbiol 62:2482–2488
Kim SH, Choi NS, Lee WI, Lee JW, Kim DH (1998) Isolation of Bacillus strains secreting fibrinolytic enzyme from Doenjang. Korean J Microbiol 34:87–90
Kim DH, Jo C, Yook HS, Park BJ, Byun MW (2002) Enhancement of preservation characteristics of Meju, an intermediate material for Korean legume-based fermented soy sauce, Kanjang, by irradiation. Radiat Phy Chem 64:317–322
Kwon GH, Lee HA, Park JY, Kim JS, Lim J, Park CS, Kwon DY, Kim YS, Kim JH (2009) Development of a RAPD-PCR method for identification of Bacillus species isolated from Cheonggukjang. Int J Food Microbiol 129:282–287
Lee KH, Jun KD, Kim WS, Paik HD (2001) Partial characterization of polyfermenticin SCD, a newly identified bacteriocin of Bacillus polyfermenticus. Lett Appl Microbiol 32:146–151
Maniatis T, Fritsch EF, Sambrook J (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Min KK, Kim MJ, Kim DH, Park YS, Kang JS (2009) Characterization of Bacillus polyfermenticus as a probiotic. J Microbial Biotechnol. doi:10.4014/jmb.0903.0113
Nagai T, Koguchi K, Itoh Y (1997) Chemical analysis of poly-γ-glutamic acid produced by plasmid-free Bacillus subtilis (natto): evidence that plasmids are not involved in poly-γ-glutamic acid production. J Gen Appl Microbiol 43:139–143
Nestel P (2002) Role of soy protein in cholesterol-lowering: how good is it? Arterioscler. Thromb Vasc Biol 22:1743–1744
Ouoba LII, Rechinger KB, Diawara B, Traore AS, Jakobsen M (2003) Degradation of proteins during the fermentation of African locust bean (Parkia biglobosa) by strains of Bacillus subtilis and Bacillus pumilus for production of Soumbala. J Appl Microbiol 94:396–402
Puska P, Korpelainen V, Hoie LH, Skovlund E, Lahti T, Smerud KT (2002) Soy in hypercholesterolaemia: a doubleblind, placebo-controlled trial. Eur J Clin Nutr 56:352–357
Reddy NR, Pierson MD, Salunkhe DK (1986) Legume-based fermented foods. CRC Press, Inc., Boca Raton, pp 1–68
Reysenbach AL, Giver LJ, Wickham GS, Pace NR (1992) Differential amplification of rRNA genes by polymerase chain reaction. Appl Environ Microbiol 58:3417–3418
Sumi H, Hamada H, Nakanishi K, Hiratani H (1990) Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Acta Haematol 84:139–143
Suvarna VC, Boby VU (2005) Probiotics in human health: a current assessment. Curr Sci 88:1744–1748
Tanimoto H, Mori M, Motoki M, Torii K, Kadowaki M, Noguchi T (2001) Natto mucilage containing poly-gamma-glutamic acid increases soluble calcium in the rat small intestine. Biosci Biotechnol Biochem 65:516–521
Turpie AG, Chin BS, Lip GY (2002) Venous thromboembolism: treatment strategies. Br Med J 325:948–950
Xu D, Cote JC (2003) Phylogenetic relationship between Bacillus species and related genera inferred from comparison of 3’ and 16S rDNA and 5’ end 16–23S ITS nucleotide sequences. Int J Syst Evol Microbiol 53:695–704
Yang T, Jia M, Mei Q, Shang P (2002) Effects of Angelica polysaccharide on blood coagulation and platelet aggregation. Zhong Yao Cai 25:344–345
Acknowledgments
This paper was supported by research funds of Chonbuk National University in 2007. We thank the Research Institute of Bioindustry at Chonbuk National University for kindly providing the facilities and materials for this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mo, AY., Kwon, B., Kamala-Kannan, S. et al. Isolation and characterization of Bacillus polyfermenticus isolated from Meju, Korean soybean fermentation starter. World J Microbiol Biotechnol 26, 1099–1105 (2010). https://doi.org/10.1007/s11274-009-0276-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-009-0276-z