Abstract
In situ bioremediation (ISB) is a widely accepted method for eradicating petroleum hydrocarbons (PHCs) and chlorinated aliphatic hydrocarbons (CAHs) from contaminated aquifers. To achieve full removal of all toxic compounds that originated from microbial degradation, sequential anaerobic/aerobic bioremediation systems are recommended. While several works based on laboratory analyses targeting sequential bioremediation have been documented, examples of sequential ISB are limited. The purpose of this study is to report and analyze the results obtained from the multiscale characterization activities propaedeutic to the construction of Italy’s largest (> 400 m long) sequential ISB system. The rich wealth of information produced during this study provides a useful example that can be followed for the construction of new sequential ISBs. The system was set up to remediate a solute plume containing PHCs and CAHs in an alluvial aquifer in Italy. Microcosm experiments were carried out to determine the biodegradation potential under anaerobic and aerobic conditions. In situ tests were performed by installing two 40-m-long pilot biobarriers for sequential anaerobic and aerobic degradation intercepting part of the contamination plume. These experiments pointed out the need of adding biostimulating compounds to accelerate the biodegradation process, under both aerobic and anaerobic conditions. In situ tests showed removal efficiencies of up to 95 and 99% for total CAHs and PHCs, respectively, proving the feasibility of the full-scale ISB system. Apparent discrepancies between laboratory and in situ tests can be ascribed to scale effects and aquifer heterogeneities.
Similar content being viewed by others
1 Introduction
Bioremediation is a widely adopted solution to eradicate petroleum hydrocarbons (PHCs) (Ismail et al., 2022; Lang et al., 2016), chlorinated aliphatic hydrocarbons (CAHs) (Essabri et al., 2019) or metal(loid)s (Carlos et al., 2016) from polluted waters. In situ bioremediation (ISB) is an application of bioremediation that relies on the natural microbial metabolic activities occurring in a polluted soil (Madsen et al., 1991). ISB aims at achieving an effective aquifer clean-up (Ismail et al., 2022; Suthersan et al., 2017) by means of the mineralization of organic compounds, organohalide respiration (OHR), redox reactions or other processes. Biostimulation aims at enhancing ISB by maximizing the activity of native microorganisms in a soil (Thomas & Ward, 1989). This is achieved through a variety of methods, such as the addition of nutrients or electron donors/acceptors and the control of subsurface environmental conditions like pH, redox state, or temperature (Majone et al., 2015).
Polluted aquifers are usually affected by a wide range of organic contaminants, which can undergo different degradation pathways. It is well known, for instance, that hydrocarbons tend to degrade under both aerobic (Lee et al., 1988) and anaerobic (Edwards & Grbić-Galić, 1992) conditions. The PHCs are usually best degraded via aerobic biodegradation (Fritsche & Hofrichter, 2004). Less frequently, hydrocarbons undergo anaerobic biodegradation (Vogt et al., 2011). Dechlorination of CAHs, such as tetrachloroethylene (PCE) or trichloroethylene (TCE), typically takes place more efficiently via OHR under anaerobic conditions (Aulenta et al., 2006). However, less-chlorinated CAHs produced by degradation of highly chlorinated CAHs are typically recalcitrant under anaerobic conditions and more easily biodegraded under aerobic conditions. Such is the case of vinyl chloride (VC) (Davis & Carpenter, 1990), which is even more toxic than the more-chlorinated compounds and is classified by the International Agency for Research on Cancer (IARC) as a cancerogenic compound.
Sequential anaerobic/aerobic bioremediation is a relatively new technique that can be adopted to achieve complete mineralization of chlorinated pollutants (Frascari et al., 2015). Essentially, it forces polluted waters to undergo a two-stage bioremediation, a first stage under reductive conditions and a second stage under oxidizing conditions. Although sequential ISB is theoretically promising and well-studied in the laboratory, examples of real-life applications have been scarcely documented (Devlin & Parker, 1996; Tiehm & Schmidt, 2011). This can be readily ascribed to the higher complexity (and costs) of a double ISB compared to a relatively simpler single ISB.
In this work, we report the analysis of the rich wealth of data obtained during the multiscale experimental activity, propaedeutic to the construction of Italy’s largest (> 400 m) sequential ISB system. Our goal is to improve the current state of the art in the design and implementation of large-scale sequential bioremediation interventions in polluted aquifers using multi-scale experimental activities. The methods systematically presented in this work can serve as an example for future applications of ISB systems, in particular when different contaminants coexist in a site and require different redox conditions to be properly degraded through ISB. Indeed, the extensive dataset and the successful results of this wide-scale characterization were critical to obtain the authorizations for the construction of the full-scale barrier by local regulators. We ultimately point out that the full-scale biobarriers are currently operating and under monitoring. The results of such monitoring will be presented in follow-up studies.
2 Background Information
2.1 Plume Source and Aquifer Configuration
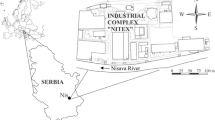
The site layout is displayed in Fig. 1. The site is bounded to the south by a lagoon, and to the north by the agricultural land reclamation canal (ALRC) (Fig. 1a), an artificial drainage system built specifically to allow agricultural activities on the land surface. The ALRC is connected to a pumping system that keeps the water level inside the canal at constant value of about − 2 m above the sea level (a.s.l.). Figure 1b provides a conceptual geological cross section of the study area (cross section A-A’ in Fig. 1a), which also shows the salient hydrogeological features of the site. Except for the top parts of the landfill, the whole area (including the contaminated aquifer) is located between 0 and − 1 m a.s.l. (i.e., below the sea level).
(a) Map of the landfill. (b) Schematic geological cross section (A-A’) of the study area. The black arrows indicate the mean groundwater flow direction, which is oriented from south-east to north-west. (c) Layout of the pilot biobarriers for the aerobic (left) and anaerobic (right) biodegradation test. The blue arrow indicates the expected flow direction. PM: observation piezometers; INJ: injection wells; EXT: extraction wells; AS: air sparging wells. ALRC = agricultural land reclamation canal. Vertical axis represents the topographic elevation above the sea level (a.s.l.)
From a geological point of view, the subsurface is characterized by an alternation of sandy and silty clay horizons. The local lithological characteristics of the subsurface horizons can be summarized as follows.
-
From the ground level down to about 1–3 m depth: silty clays and clayey silts.
-
From 1–3 m down to about 9–12 m depth: medium-size sand lenses (forming the “shallow aquifer”) embedded into a silty fine sand and sandy silt matrix.
-
From 9–12 m down to about 20–22 m depth: dense alternation of clays, silts, and silty clays, intercalated with more or less silty sands (“intermediate aquifers”).
-
From 20–22 m down to about 34–36 m depth: dense alternation of silty clays and lenticular decimeter levels consisting of clayey silts and silts with fine sand components.
-
From 34–36 m down to 50–51 m depth: alternation of sand and silt soils with sandy soils (“deep aquifers”).
The hydraulic conductivity (K) of the sandy horizons constituting the main aquifers is close to \(K={10}^{-4}\) m/s, based on the slug and pumping tests. The silt and clay horizons are aquitards/aquicludes with low to very low hydraulic conductivity (\(K<{10}^{-7}\) m/s). Depending on the spatial continuity of the fine-grained top layer, the shallow aquifer can be either unconfined or semi-confined, similar to that reported by Dalla Libera et al. (2021) in another adjacent site with similar hydrogeological behavior. The shallow aquifer is more vulnerable to the contamination that originated from the ground surface than the intermediate and deep aquifers, which tend to be more confined and therefore better protected from contamination sources.
The piezometric level of the shallow aquifer is close to the ground surface. Groundwater flows from the lagoon to the mainland, crossing the landfill, as schematically depicted by the blue arrows in Fig. 1a. The lagoon and rainfall events recharge the aquifer, which is drained by the ALRC. Groundwater head levels are affected by daily tide variations. The average hydraulic gradient (i) is close to i = 0.1%, with minor variations throughout the year. All piezometers show a similar trend in groundwater head levels, with winter minima and summer maxima.
Groundwater in the shallow aquifer crossing the landfill is likely in direct contact with the landfill waste. Groundwater is therefore enriched in the solute-form byproducts linked to the landfill’s leachate losses. Solutes are transported towards the ALRC, forming an extended plume of pollutants in the shallow aquifer, which is the target of the bioremediation system. The groundwater quality between the landfill and the canal is monitored through an extended network of boreholes screened in the shallow aquifer as well as through the P&T system. Table 1 provides a summary of the aqueous concentration of selected compounds sampled in May 2013. The concentrations of aromatic hydrocarbons, such as BTEX or monochlorobenzene (MCB), and chlorinated aliphatic hydrocarbons (CAHs), such as PCE, TCE, dichloroethenes (1,1-DCE, cis-1,2-DCE, trans-1,2-DCE), VC and chlorinated ethanes, as well as metals like As, Fe and Mn, largely exceed the Maximum Contaminant Level (MCL) set by the Italian law (Table 1).
The landfill responsible for the shallow aquifer contamination is a historical waste disposal site which was closed in the 1970s. The plume was first detected in 2001, when high concentrations of CAHs and PHCs were observed outside the landfill. These observations prompted a number of interventions, which included the installation of a pump-and-treat (P&T) system outside the landfill. The P&T consists of seven wells close to the landfill and seven wells along the ALRC (Fig. 1a). The latter wells are still active nowadays. Pumped water is treated ex situ through a sequential combination of filtering and precipitation, air stripping and catalytic oxidation and activated carbon adsorption. Although the P&T system was useful for the hydraulic confinement of the site, it was not efficient for its cleanup. This is not uncommon, as P&T systems entail long remediation times as well as elevated operational and management costs (Casasso et al., 2020; EPA, 2001; Pedretti, 2020). New investigations started in 2005–2006 to prepare an overall plan for site clean-up by selecting the best intervention technology. This is when the idea of an ISB was conceived as a viable cost-effective alternative to the P&T, eventually leading to the experimental activities described in this paper.
2.2 Assessment of Degradative Microbial Communities
A preliminary molecular analysis based on quantitative real-time polymerase chain reaction (q-PCR) was conducted on a groundwater sample (2 L) obtained by mixing equal volumes of water from two piezometers. Such piezometers, now dismissed, were located in the proximity of the aerobic and anerobic pilot area. The purpose of the analysis was to prove the biodegradation potential of the site by providing a first-cut estimation of the indigenous bacterial population able to conduct OHR. Detailed information about the specifics related to the experimental analysis is provided as Supplementary Information (S1).
The results showed that the total amount of bacteria was in the order of 3.5 × 105 (± 1.75 × 105) 16S rRNA gene copies mL−1. OHR bacteria Dehalobacter restrictus and Dehalococcoides ethenogenes were 3.13 × 100 (± 3.33 × 10−1) and 1.68 × 102 (± 8.07 × 101) gene copies mL−1, respectively. Genes for lower-chlorinated ethenes reductases bvcA and vcrA were identified, being 2.65 × 102 (± 5.38 101) and 7.18 × 102 (± 1.95 × 102) gene copies mL−1, respectively.
These data indicate that specific bacteria involved in the process of OHR are present at the site. Both Dehalobacter restrictus and Dehalococcoides ethenogenes are known to conduct OHR of CAHs. Dehalobacter restrictus is known to dechlorinate higher CAHs, such as PCE and TCE (Holliger et al., 1998). Dehalococcoides ethenogenes can also transform lower CAHs, such as DCE and VC, to ethene (Holliger et al., 1998). Although concentrations were clearly too low to sustain an efficient natural attenuation (Lendvay et al., 2003), the presence of specific bacteria involved in the process of OHR suggested the potential of the natural microbial community to sustain bioremediation of CAHs at the site, if properly stimulated.
3 Experimental Activities
In this study, we focused on two experimental activities that were carried out in the site. Firstly, microcosm experiments were performed to evaluate the biodegradation capabilities of the indigenous bacterial community under natural and biostimulated aerobic and anaerobic conditions. Secondly, the efficiency of coupled anaerobic and aerobic biodegradation in the field was evaluated by setting-up two 40-m-long pilot biobarriers (Fig. 1c).
3.1 Microcosms
Microcosms were prepared to evaluate anaerobic and aerobic degradation in the presence of native microbial communities, able to perform OHR of CAHs and oxidation of PHCs. Addition of reducing substrates, oxygen and nutrients (phosphorus and nitrogen) was performed to assess the potential benefit of biostimulation on the above-mentioned microbial activities.
After stabilization of physical parameters, groundwater samples were withdrawn from three boreholes located in different parts of the plume, put into sterile glass bottles and kept refrigerated upon arrival to the laboratory. Aquifer sediment samples were collected (at a depth of − 4.5 m a.s.l.) after drilling procedure, by choosing the internal portion of the sediment core to avoid contamination.
Anaerobic microcosms were prepared under anaerobic glove box (N2/H2/CO2, 85:10:5). Glass serum bottles (120 mL) were filled with 70 mL groundwater:sediment phase (50:50 [v/v]) and sealed with Teflon®-coated septa. Where appropriate, reducing substrates (molasses, cheese whey and soy oil) were added to a final TOC of 180 mgC L−1.
Aerobic microcosms were prepared with 70 mL of groundwater in glass serum bottles (120 mL) sealed with Teflon®-coated septa. To prevent volatilization of organic compounds, water was transferred from sampling bottle to serum bottles by gas-tight peristaltic pump. Along with unamended microcosm, oxygen and oxygen and nutrients (phosphorus and nitrogen) amended microcosms were prepared.
Microcosms were incubated at RT for 9/14 months. Abiotic microcosms were also prepared for each condition. All the study was conducted on triplicate samples. Benzene, MCB, toluene and CAHs were analyzed by gas chromatography mass spectrometry (GC–MS) method at VITO laboratories (Boerateng, Belgium). Analyses were conducted on headspace gas, sampled by static headspace sampling method (APAT, 2003). A 5975B Gas Chromatograph-Mass Spectrometer (Agilent Technologies, Santa Clara, CA, USA) equipped with D3792 PoraBOND Q column (25 m × 0.32 mm, 5.00 µm) (Agilent Technologies, Santa Clara, CA, USA) was used. Helium at 250 °C was the carrier gas. Samples were injected manually with a split ratio of 3:1 and a split flow of 7.5 mL. Oven temperature conditions were 40 °C for 2 min and then 10 °C/min to 260° for 7 min. Arsenic was analyzed by the ICP-MS method on filtered (45 µm Millipore filters) water samples (Corsini et al., 2014). The determination of volatile fatty acids was carried out with GC-FID, after their extraction from groundwater samples with diethyl ether, as described in Vanbroekhoven et al., 2007.
3.2 Pilot Biobarriers
Two pilot biobarriers were installed and monitored to evaluate in situ aerobic and anaerobic bioremediation processes. The anaerobic biobarrier was created in the proximity of the landfill, while the aerobic biobarrier was created hydraulically downgradient of the anaerobic site and in the proximity of the ALRC (Fig. 1a). The layout of each biobarrier is shown in Fig. 1c. The two biobarriers were installed along the same hypothesized groundwater flow path, such that the upgradient anaerobic barrier triggered anaerobic bioremediation, while the downgradient site targeted aerobic degradation. A key hypothesis was that the downstream aerobic barrier intercepts and degrades the plume of lower CAHs, forming as a consequence of the incomplete dechlorination of the higher CAHs occurring in the upstream anaerobic barrier. In the case of chloroethenes, the dechlorination of PCE and TCE occurs mainly via OHR under anaerobic conditions, producing DCE and VC, whose degradation is favored under aerobic conditions. The sites of the two pilot biobarriers were therefore chosen such that:
-
Anaerobic conditions were enhanced close to the contamination source (the landfill) by injecting a reducing substrate, to promote the degradation of more-chlorinated compounds;
-
Aerobic conditions were enhanced downgradient of the source and in the proximity of the ALRC, to promote the degradation of less-chlorinated compounds (mainly VC) produced by the activity of the anaerobic biobarrier.
The anaerobic biobarrier included four observation boreholes (labeled as “ANPM” in Fig. 1c), two pumping wells for groundwater extraction (“EXTAN”) and three wells for reinjection of the extracted water (“INJAN”) amended with a reducing substrate to stimulate microbial activity in the aquifer. A total volume of 5.5 m3 of substrate-dosed water was injected into the ground during the 475 testing days by continuous injection through wells INJAN1, INJAN2 and INJAN3, corresponding to a total injected COD of 3575 kg. To evaluate the effectiveness of the biostimulation process, two piezometers (ANPM1, ANPM2) were located upgradient of the injection-extraction wells and two piezometers (ANPM3, ANPM4) were located downgradient. The inter-axis between wells was about 10 m.
The aerobic barrier included four observation boreholes (labeled as “AEPM” in Fig. 1c), four air sparging wells (“ASAE”) for oxygen injection, two extraction wells (“EXTAE”) for groundwater withdrawal and three injection wells (“INJAE”) for reinjection of water amended with nutrients (nitrogen and phosphorus-containing compounds). Specifically, each AS well injected at a rate of 1600 L h−1, resulting in a total cumulative injected air volume of about 73,000 m3 and a total injected mass of oxygen of about 18,800 kg. The total volume of recirculated water was about 1100 m3, with an average flow rate of about 10 L h−1. The total mass of injected nitrogen and phosphorus was about 93 kg and about 38 kg, respectively.
All boreholes were drilled using destructive core methods, except for ANPM1 and AEPM1, where continuous cores were obtained. The drillings reached the low-permeability layer at the bottom of the shallow aquifer at about 10 m depth. The well and piezometer cases were made of HDPE, respectively of 3″ and 4″ diameters.
The tests were conducted between June 2013 and September 2014. A first monitoring survey of the pilot sites was performed in May 2013 in order to assess the contamination prior to the start of biostimulation activities (Table 1). Subsequently, monitoring campaigns were carried out with an average frequency of 45 days to evaluate the effectiveness of the biodegradation processes. In each monitoring survey, concentrations of key contaminants, dissolved oxygen, oxidation–reduction potential (ORP), temperature, electrical conductivity and pH were measured. The well-known statistical Mann–Kendall test was used to verify the presence of trends in the concentration time series collected in the different experiments.
Ultimately, benzene-, chlorobenzene-, toluene-, VC-, and DCA-degrading bacteria active in the aerobic pilot test were determined through the “most probable number” (MPN) method. Samples collected from piezometers located inside and outside the aerobic biobarrier were analyzed and compared. Details about the underpinning methods are provided as Supplementary Information (S2).
4 Results
4.1 Laboratory Analysis
4.1.1 Anaerobic Microcosm Experiments
The anaerobic microcosms showed an overall efficient biodegradation of CAHs (Fig. 2) under both natural and biostimulated conditions, although the addition of nutrients significantly enhanced biodegradation. In all microcosms, pH remained at circumneutral values after 4 and 9 months. The ORP (expressed as Eh) in the unamended microcosms was around − 200 mV. Cheese whey amended microcosms reached the lower ORP (− 350 mV), followed by the molasses and soy oil amended microcosms (− 300 mV). In abiotic microcosms, ORP was around − 35 mV. In unamended microcosms (which reflect the natural attenuation capability of the aquifer), high yields of TCE, cis-1,2-DCE, 1,1-DCE and 1,1,2-TCA biodegradation were reached after 9 months, although concentrations were still much higher than the MCL for each of these contaminants (several tens of µg L−1). For 1,2-DCE the law limit is imposed for the sum of cis- and trans-1,2-DCE at 60 µg L−1. Therefore, even if the concentration of cis-1,2-DCE after 9 months was equal to 20 µg L−1, the concentrations of trans-1,2-DCE at 9 months (above 300 µg L−1) still exceeded the MCL.
Main results from the anaerobic microcosms. “Natural attenuation” refers to unamended microcosms; “Abiotic” and “Soy oil (ab.)” are the control microcosms for unamended and amended microcosms, respectively; “Molasses”, “Soy oil” and “Cheese whey” refer to the amended microcosms. Incomplete time series are due to missing data not communicated by the laboratory performing the analyses
Biostimulating the microcosms with the addition of nutrients increased the biodegradation rates up to five times compared to the natural degradation rates. Figure 2 shows that biostimulation helped reaching an almost complete removal of most of the studied compounds (concentrations < 5 µg L−1) after 2 months. Instead, such results could only be reached after 10 or more months with natural attenuation.
Specifically, trans-1,2-DCE, 1,1-DCA and 1,2-DCA proved to be more recalcitrant. Scarce biodegradation was observed in unamended microcosms (meaning that natural attenuation in the site was not efficient), while the addition of substrates was able to enhance the degradation of trans-1,2-DCE and 1,2-DCA. While significant drops in concentrations were observed for these two compounds, they still remained in the order of several hundred µg L−1. 1,1-DCA was not degraded at all, not even under biostimulated conditions. 1,1,2,2-TeCA concentrations could not be determined during the experiments due to analytical interferences. The addition of substrates produced a dramatic variation in VC response. In the amended microcosms, concentrations dropped from about 3000 µg L−1 to values under 5 µg L−1 within 4 months. In the unamended microcosms, an accumulation of VC took place, with concentrations eventually reaching > 6000 µg L−1.
The addition of substrates increased methane production. In microcosms amended with molasses and soy oil, methane concentrations increased from about 650 µg L−1 to about 800 µg L−1. In the microcosms with cheese whey addition, concentrations above 2000 µg L−1 were reached. No increase in arsenic was observed. An increase in volatile fatty acids, particularly acetic acid (CH3CO2H), was observed after 4 months in the microcosms with the addition of soy oil and cheese whey (from less than 0.5 mg L−1 to tens of mg L−1). These acids are a product of organic substrates fermentation as well as byproducts of contaminants biodegradation. Moreover, they can be further fermented to produce hydrogen which is used in the OHR processes.
4.1.2 Aerobic Microcosm Experiments
The results of aerobic microcosm experiments are shown in Fig. 3. VC was efficiently degraded with and without the addition of nutrients under aerobic conditions. However, the addition of nutrients sped up the reaction, leading to an almost complete removal of VC after only 28 weeks in the amended microcosms, compared to 41 weeks required in unamended microcosms. Similar benefits of nutrients were observed for the biodegradation of cis-1,2-DCE, although no complete removal of this compound was observed at the end of the experiments (concentrations of almost 1 mg L−1 were still observed after 41 weeks). The concentrations of other CAHs such as 1,1-DCA, 1,1-DCE, trans-1,2-DCE, TCE and PCE did not decrease significantly under aerobic conditions, not even in amended microcosms (data not reported). An accumulation of 1,2-DCA was observed, leading to an increase of concentrations with time. This effect resulted stronger with addiction of oxygen and nutrients. Aerobic conditions were able to reduce the concentration of benzene by 33% (i.e., from about 300 µg L−1 to about 200 µg L−1) at week 28, with a rebound at week 41. The behavior of benzene was not affected by the addition of oxygen nor by the addition of oxygen and nutrients. Similar results were observed for MCB, whose concentrations were reduced by about 25% (i.e., from about 400 µg L−1 to about 300 µg L−1) at week 28.
4.2 Pilot Biobarriers
4.2.1 Anaerobic Biodegradation
The concentrations of PCE, its degradation products, TCA and 1,2-DCA at the anaerobic biobarrier are shown in Fig. 4. During the experimental time, PCE concentrations at ANPM4 (one of the piezometers downgradient of the injection wells) decreased by nearly 100%, reaching values below the MCL. According to the Mann–Kendall test, ANPM3 (the second downgradient piezometer) showed a negative trend in concentrations too, which confirmed the degradation of this compound induced by the anaerobic pilot barrier, although with different degrees of efficiency. On the other hand, Mann–Kendall tests indicated that no statistically significant trend was observed for piezometers ANPM1 and ANPM2 located upstream of the injection wells, as expected, since they represent the concentrations entering the biobarrier. Similar trends were observed for TCE, 1,1-DCE and VC. For this last compound, a decrease in concentrations up to three orders of magnitude was observed in the downstream piezometer ANPM4. Nonetheless, VC concentrations remained much above the MCL. For TCA and 1,1-DCA, the Mann–Kendall test showed no statistical significance for the two upgradient piezometers, while the opposite was found for the two piezometers downgradient of the wells, which showed a progressive decrease in concentrations with time. For TCA, concentrations dropped by about 96.8% between the beginning and the end of the experiments. For 1,1-DCA the drop was close to 97.8%. For benzene and MCB, no statistically valid trends were detected in the upgradient piezometers and in the downgradient piezometer ANPM3. At ANPM4, negative trends for both compounds were instead observed.
The variation of environmental parameters, concentrations of major ions and dissolved oxygen at the up- and downgradient piezometers (Fig. 5) was consistent with the degradation of CAHs. The oxidizing-reduction potential (ORP), expressed in terms of Eh, dropped from near-zero values (i.e., a system close to oxidizing conditions) to much more negative values, suggesting that reducing conditions were established in the aquifer within the anaerobic pilot area. The more pronounced change in ORP at ANPM4 (up to − 300 mV) agrees well with larger reduction in concentrations (i.e., more efficient degradation) of CAHs at this piezometer compared to the other piezometers (Fig. 4). Total iron concentrations were also observed to progressively decrease with time, particularly at ANPM4.
4.2.2 Aerobic Biodegradation
The data collected during the pilot test demonstrated that aerobic conditions could provide great reductions of BTEX, MCB and VC concentrations, as well as other monitored contaminants (Fig. 6). In certain piezometers, the overall reduction of compounds such as benzene, p-xylene, MCB and VC was > 99%. The reduction led to concentrations well below the MCL. For VC, in situ observations at AEPM4 were consistent with the results obtained in the aerobic microcosms (Fig. 3), while for benzene and MCB in situ biodegradation was more effective. The maximum decrease in concentrations was found at AEPM4, one of the two piezometers located upgradient of the injection zone. Of the two piezometers located downgradient of the injection zone, only AEPM2 provided a reduction in concentrations with time. The other piezometers, AEPM1 and AEPM3, did not provide a significant decrease in concentrations with time, as corroborated by the Mann–Kendall test.
At AEPM4, a substantial reduction in the concentrations of metal(loid)s (As, Fe, and Mn) was also reported during the test (Fig. 7), compared to the initial concentrations before aerobic conditions were established in the pilot area. A steady decrease of As and Fe concentrations was observed until values under the MCL were reached. Similar trends were detected in the early stages of the test performed at AEPM2, although a concentration rebound was observed by the end of the experiment. Concentrations above the MCL were instead maintained in piezometers AEPM1 and AEPM3.
At AEPM3 (upgradient of the aerobic pilot site), toluene- and chlorobenzene-degrading bacteria were present in the order of 102 most probable number (MPN) g−1 soil dry weight (d.w.), whereas benzene-, VC, 1,2-DCA and 1,1-DCA-degrading bacteria were one order lower. At EXTAE2 (downstream of the injection piezometers), the MPN of degrading bacteria increased by one order of magnitude in the presence of all the tested compounds, and by two orders in the presence of toluene. These results indicate that the natural aquifer bacterial community hosts active degrading bacteria which were stimulated by the aerobic treatment.
5 Analysis and Discussion
5.1 Laboratory Tests
The analysis of results from unamended anaerobic microcosms confirmed the molecular survey data regarding the poor effectiveness of natural attenuation at the site. TCE, cis-1,2-DCE, 1,1-DCE and 1,1,2-TCA were slowly transformed, while the ability of bacteria to dechlorinate trans-1,2-DCE, VC, 1,1-DCA and MCB without biostimulation proved to be poor or non-existent. This is a common drawback pointed out in several works at all scales — microcosm and in situ (Fennell et al., 2001). This behavior might be associated with the lack of sufficient number of microorganisms in water samples or to the competition of microorganisms for electron donors. The addition of reducing substrates led to a more efficient and faster dechlorination of all CAHs, consistently with observations from other sites (Courbet et al., 2011; Lendvay et al., 2003). The only exceptions were 1,1-DCA and MCB, which is also in accordance with previous reports (Matteucci et al., 2015).
Aerobic conditions contributed to the degradation of cis-1,2-DCE, 1,1-DCE and VC, which were produced by dechlorination of chloroethenes PCE and TCE. Aerobic conditions were therefore needed to avoid the stall of less-chlorinated compounds in the site. Aerobic conditions were also able to degrade contaminants that were not efficiently transformed under anaerobic conditions, such as benzene. On the contrary, 1,1-DCA degradation remained limited even under aerobic conditions. As observed in other studies (Kao et al., 2003), oxygen supply was useful to sustain aerobic degradation of contaminants, while the addition of nutrients helped speed up the process.
5.2 Pilot Biobarriers
At the anaerobic biobarrier, monitored concentrations of CAHs (Fig. 4) were generally higher in each upgradient piezometer (ANPM1 and ANPM2) compared to the respective downgradient piezometer (ANPM4 and ANPM3, respectively). The gap between upgradient and downgradient concentrations for each contaminant became increasingly larger with time. Lack of statistically significant (negative or positive) trends in PCE, TCE, 1,1-DCE, cis- and trans-1,2-DCE and VC concentrations with time was also observed in piezometers ANPM1 and ANPM2 (upgradient of the injection wells). The discrepancies between up- and downgradient piezometers can be explained considering that the soil surrounding the upstream piezometers did not receive the hydrogen-releasing substrate that promotes microbial activity. This agrees well with the hypothesis that the compounds dissolved in groundwater upgradient of the injection wells were less prone to undergo biodegradation than the compounds found downgradient of the wells.
The decrease of PCE and TCE concentrations to values near the MCL (1.1 µg L−1 and 1.5 µg L−1, respectively) in the piezometers downgradient of the injection wells (ANPM3 and ANPM4) proved the degradation efficiency of the ISB with respect to the higher chlorinated ethenes. The accumulation of DCE and VC, instead, was consistent with the fact that anaerobic conditions were not as favorable for less-chlorinated ethenes dechlorination. This further demonstrated the need to create a sequential anaerobic/aerobic ISB system to remove both high- and less-chlorinated compounds.
5.3 Role of Redox Changes
The injection of water dosed with reducing substrate in the anaerobic pilot site generated a drop in Eh values, which were critical to interpret the variation in observed concentrations.
For instance, the more pronounced drop in concentrations of CAHs at ANPM4 (Fig. 4) can be explained considering the larger drop in Eh (i.e., more reducing conditions) at this piezometer compared to the others (Fig. 5). Reducing conditions are achieved via fermentation of the organic substrate carried out by microorganisms, which leads to hydrogen and small organic acids production. Hydrogen acts as an electron donor in anaerobic microbial activities that first consume oxygen dissolved in groundwaters. After depletion of dissolved oxygen, anaerobic microbes use nitrate as a terminal electron acceptor, followed by manganese (IV), iron (III), sulfate, and finally carbon dioxide (methanogenesis) leading to more and more reducing conditions in the aquifer.
The strong decrease of sulfate concentrations may indicate that sulfate-reducing conditions have been reached, suggesting that anaerobic dechlorination can take place efficiently. The reduction of sulfates is coupled to a decrease in iron concentrations in groundwater, possibly as the result of (chemical or microbiological mediated) precipitation of iron sulfides, which is a common occurrence during in situ anaerobic bioremediation of chlorinated hydrocarbons (such as enhanced OHR) due to availability of both free sulfide and Fe2+ (He et al., 2015; Hyun & Hayes, 2009). The occurrence of this process is also supported by another fact: if the free sulfides produced by sulfate reduction were not removed from groundwater, they would inhibit biotic OHR, which is instead an ongoing process.
In particular, sulfate reduction operated by sulfate reducing bacteria increases pH (Luptakova et al., 2013; Mukwevho et al., 2019), giving a possible explanation for the positive trend observed for pH values. At the same time, iron sulfides may also cause abiotic dechlorination of CAHs, contributing to the overall degradation taking place in situ (e.g., He et al., 2015; Jeong & Hayes, 2007; Tobiszewski & Namieśnik, 2012).
No trends for As concentrations were statistically detected. However, under reducing conditions, As precipitation during microbial sulfate reduction appears to be important in determining its mobilization or immobilization (Kirk et al., 2004). Sulfides produced by sulfate reduction can form minerals that remove As from solution (Moore et al., 1988; Rittle et al., 1995). These include pure As sulfides (e.g., orpiment and realgar) (Burton et al., 2014) or Fe(II) sulfides (e.g., troilite and pyrite) (Bostick & Fendorf, 2003; Bostick et al., 2003). The As removal capability of Fe(II) sulfides is directly linked to the mineral types (Burton et al., 2014; O’Day et al., 2004) and the interactions between As and those kinds of sulfide minerals that potentially form in aquifers as well as mechanisms responsible for in situ As immobilization have yet to be understood (Kirk et al., 2010; Pi et al., 2017).
5.4 Anaerobic Degradation of Benzene and MCB
The efficiency of anaerobic degradation for benzene and MCB was surprising, although not completely unexpected. Anaerobic biodegradation of benzene and other aromatic hydrocarbons has been reported under nitrate-, iron-, manganese-, sulfate-reducing and methanogenic conditions. Comprehensive reviews on this topic are provided by, e.g., Foght (2008) and Haritash and Kaushik (2009). In situ anaerobic degradation has also been observed for chlorobenzenes (e.g. MCB), which can be achieved via OHR under both methanogenic and sulfate-reducing conditions (Liang et al., 2011). Although the transformation of MCB to benzene in a methanogenic enrichment culture on river sediments has been documented in the past (Nowak et al., 1996), PHCs degradation is normally better achieved under aerobic conditions.
5.5 Aerobic Biodegradation
As for the degradation of aromatic hydrocarbons as well as VC, the results suggested that an aerobic biobarrier was needed downstream of the anaerobic one (Fig. 6). A general reduction in contaminant concentrations was observed mainly at one piezometer upgradient of the pilot barrier (AEPM4) and at one piezometer downgradient of the barrier (AEPM2). This may suggest that the flow field in the aerobic area is not directed as expected. This could be explained by the presence of soil heterogeneities, possibly combined with the intense pumping activity of air and water injection wells, which creates local increase in groundwater levels that could cause diverging radial flow conditions. A reduction in hydraulic conductivity due to biomass growth or generation of biogenic gases may also potentially lead to preferential flow of groundwater in the subsurface.
The simultaneous decrease in VC, PHCs and ammonia concentrations (in particular at piezometers AEPM4 and AEPM2—Fig. 6) suggested that co-metabolic aerobic oxidation by hydrocarbon-oxidizing bacteria is ongoing. These bacteria use dioxygenase or monooxygenase enzymes to initiate the biodegradation process of growth substrates (“auxiliary substrates”), such as ammonium and aromatic pollutants (Dolinová et al., 2017; Frascari et al., 2015; Tiehm & Schmidt, 2011), which are present in high concentrations in the site. As oxygenases have a very broad substrate range, they could fortuitously oxidize chloroethenes, yielding unstable chlorinated epoxides that subsequently break down spontaneously. Co-metabolic degradation has been shown for all chloroethenes but it is more efficient for the less chlorinated (i.e. less oxidized) ones (e.g., Mattes et al., 2010). Moreover, aerobic metabolic oxidation of VC might also take place at the site in microaerophilic (i.e., oxygen 0.5–1.8 mg L−1) conditions (Singh et al., 2004) by VC/ethenetrophic bacteria (Liu et al., 2017).
The substantial reduction of the concentrations of metal(loid)s (As, Fe, and Mn) at the aerobic barrier, previously present in groundwater under anaerobic conditions, was an expected result of the injection of oxygen. The injection of oxidants is a well-known method for in situ treatment of metal-contaminated groundwaters. The oxidation of iron and manganese triggers the precipitation of Fe(III)-(hydr)oxides and Mn(IV)-oxides and arsenic can be removed from solution both by surface adsorption and coprecipitation with these minerals.
5.6 Scaling Effects in Bioremediation Rates
The comparison between laboratory and pilot-scale experiments suggests that bioremediation was generally slower in the field than in the laboratory. For instance, in Fig. 4, we noted that after the injection of the reducing substrate, about 4 months were needed for the bioremediation to take place in the pilot test site. In comparison, we observed a much quicker activation within the microcosms under equivalent anaerobic conditions. Such “scaling effects” in bioremediation agree well with previously documented experience from other sites (e.g. Sturman et al., 1995). Hydrogeological and biochemical heterogeneities are likely the strongest controls of scaling effects, affecting subsurface flow and transport and biochemical reactions in many ways.
Physical heterogeneities, associated to the variation of hydraulic parameters such as the hydraulic conductivity (K), control the transport rate and consequently the pore-scale residence time of groundwater. Small variations in K can lead to preferential paths and solute transport channeling (e.g. Bianchi & Pedretti, 2017), a situation which is severely exacerbated in the proximity of pumping wells (Pedretti, 2020; Pedretti et al., 2013), where flow and transport tend to be stratified. Transport channeling determines a variation in the pore water residence time. When biostimulation is performed by pumping liquid solutions into the aquifer wells, the resulting distribution of such reactive liquids in a heterogeneous aquifer could result in a non-homogeneous distribution of reactants. In turn, this could lead to a non-homogeneous biodegradation in the site and the formation of apparent scale effects of biochemical reactions. Microcosms experiments are instead performed in more homogeneous closed systems, at stable temperature and with an even distribution of biostimulating compounds in the porewater. Microcosms are also often performed for very long residence times (e.g. Aulenta et al., 2006).
Even though little information was available to quantify physical heterogeneity in the study area, Dalla Libera et al., (2020, 2021) reported strong textural and compositional variations at a relatively small (i.e. metric) scale in a site located only few km away from the one investigated in this study. Hence, such spatial variations may indeed be occurring in our study area. From a biochemical perspective, investigations are ongoing to disentangle the spatial variability of natural substrate and the distribution and types of microbiological communities.
5.7 Limitation of the Study and Future Developments
The multi-scale activities described in this work were fundamental to obtain the environmental permits for the construction of the full-scale ISB system. Such a system was set up a few years after the end of these experiments (i.e., after 2014) and is currently being monitored. Critical aspects currently being evaluated involve “secondary contamination”, created by toxic intermediates of sequential bioremediation chains (e.g., VC accumulation) and compound-specific isotope analysis, which is expected to provide a better assessment of biodegradation of chloroethenes and give further information on the efficiency of the ISB system. These results will be presented in a follow-up study.
We also foresee the setup of a model for the interpretation of combined multiscale observations and evaluate the effectiveness of the full-scale ISB system. Models are fundamental tools that can assist decision makers in predicting the behavior of a bioremediation system integrating evolving scales (Sturman et al., 1995). Models have also proven scaling-up ability, which can be useful to resolve the apparent scale dependency of parameters (e.g., kinetic rates) estimated from multiscale tests. We believe that a sound model-based analysis could be highly beneficial to break down the several non-technical barriers limiting the development of a full-scale bioremediation facility. In countries such as Italy, regulatory acceptance of in situ remediation techniques remains complicated. For instance, administrations remain skeptical, if not reluctant, towards the (re)injection of water and nutrients into polluted aquifers. Therefore, we expect that properly developed models based on solid experimental results could contribute to increase the acceptance of ISBs by administrations.
6 Summary and conclusion
This work analyzed the results from a combination of multi-scale analyses that were propaedeutic for the creation of Italy’s largest in situ sequential bioremediation (ISB) system. Monitored experiments including microcosms and in situ pilot scale biobarriers suggested that natural biodegradation and a single anaerobic biobarrier were insufficient solutions to fully degrade CAHs and PHCs. Sequential anaerobic–aerobic bioremediation barriers and biostimulation increased the effectiveness of the bioremediation products, as key contaminants achieved concentrations below the Maximum Contaminant Level (MCL) set by the Italian law. This included the less-chlorinated products (e.g., VC) forming from the degradation of highly chlorinated compounds (e.g., PCE). Specific conclusions achieved from this study are presented as follows.
In the microcosms:
-
Natural attenuation was observed as an effective degradation method only for certain compounds, such as chloroethenes degradation under anaerobic conditions. This is consistent with initial microbiological molecular surveys that detected the onsite presence of the OHR bacteria Dehalobacter restrictus and Dehalococcoides ethenogenes. In unamended microcosms, significant biodegradation of TCE, cis-1,2-DCE and 1,1-DCE was achieved after 9 months, although concentrations remained much higher (several tens of µg L−1) than the MCL. Moreover, an accumulation of the more toxic daughter product VC (up to 7000 µg L−1) was observed.
-
Biostimulation in the microcosms had a strong control on degradation kinetics. In the amended anaerobic microcosms, TCE, 1,1-DCE and cis-1,2-DCE concentrations dropped from several hundreds or thousands of µg L−1 to values under 5 µg L−1 within 2 months, i.e., at a much faster rate than in the unamended microcosms. Biostimulation was effective also on VC, as its concentrations were reduced from 4000 µg L−1 to less than 5 µg L−1 in only 4 months.
-
Aerobic microcosms showed a decrease of hydrocarbon concentrations regardless of the addition of nutrients. As for CAHs, the most important result was achieved for VC, which was efficiently degraded within 7 months with the addition of nutrients (concentrations dropped from almost 40,000 µg L−1 to about 10 µg L−1). Noteworthy results were also achieved for cis-1,2-DCE, whose concentrations dropped from more than 8000 µg L−1 to less than 1000 µg L−1 in 7 months with the addition of nutrients.
In the pilot biobarriers:
-
The anaerobic tests showed consistent results compared to the anaerobic microcosms. An efficient degradation was observed for chloroethenes. PCE concentrations were reduced almost by 100%, reaching values below the Italian MCL. A significant reduction of TCE, 1,1-DCE and VC concentrations was observed, although concentrations never dropped below the corresponding MCLs. In particular, VC concentrations decreased from about 132,000 µg L−1 to about 190 µg L−1, i.e., up to three orders of magnitude.
-
The aerobic pilot test demonstrated the efficiency of in situ aerobic degradation of PHCs. Remarkably, VC concentrations were reduced from about 65,000 µg L−1 to about 2 µg L−1. The aerobic barrier also helped in degrading benzene and MCB; in certain piezometers, concentrations of these compounds were reduced from about 200 µg L−1 and about 500 µg L−1, respectively, to < 1 µg L−1. In situ biodegradation of benzene and MCB was more effective than in the microcosms.
Data availability
All data generated or analyzed during this study are included in this published article and the supplementary information file.
References
APAT. (2003). APAT, Protection agency of environment and technical services. Analytical methods for chemical water analysis, vol. 1. (“Metodi analitici per le acque - Volume I - Sezione 1000”. APAT Rapporti 29). IRSA-CNR (Institute for Water Research, National Research Council). In Italian.
Aulenta, F., Majone, M., & Tandoi, V. (2006). Enhanced anaerobic bioremediation of chlorinated solvents: Environmental factors influencing microbial activity and their relevance under field conditions. Journal of Chemical Technology & Biotechnology, 81(9), 1463–1474. https://doi.org/10.1002/jctb.1567
Bianchi, M., & Pedretti, D. (2017). Geological entropy and solute transport in heterogeneous porous media. Water Resources Research, 53(6), 4691–4708. https://doi.org/10.1002/2016WR020195
Bostick, B. C., & Fendorf, S. (2003). Arsenite sorption on troilite (FeS) and pyrite (FeS2). Geochimica Et Cosmochimica Acta, 67(5), 909–921. https://doi.org/10.1016/S0016-7037(02)01170-5
Bostick, B. C., Fendorf, S., & Manning, B. A. (2003). Arsenite adsorption on galena (PbS) and sphalerite (ZnS). Geochimica Et Cosmochimica Acta, 67(5), 895–907. https://doi.org/10.1016/S0016-7037(02)00959-6
Burton, E. D., Johnston, S. G., & Kocar, B. D. (2014). Arsenic mobility during flooding of contaminated soil: The effect of microbial sulfate reduction Environmental Science & Technology. American Chemical Society, 48(23), 13660–13667. https://doi.org/10.1021/es503963k
Carlos, F. S., Giovanella, P., Bavaresco, J., de Borges, C. S., & de Camargo, F. A. O. (2016). A comparison of microbial bioaugmentation and biostimulation for hexavalent chromium removal from wastewater. Water, Air, & Soil Pollution, 227(6), 175. https://doi.org/10.1007/s11270-016-2872-5
Casasso, A., Tosco, T., Bianco, C., Bucci, A., & Sethi, R. (2020). How can we make pump and treat systems more energetically sustainable? Water, 12(1), 67. https://doi.org/10.3390/w12010067
Corsini, A., Zaccheo, P., Muyzer, G., Andreoni, V., & Cavalca, L. (2014). Arsenic transforming abilities of groundwater bacteria and the combined use of Aliihoeflea sp. strain 2WW and goethite in metalloid removal. Journal of Hazardous Materials, 269, 89–97. https://doi.org/10.1016/j.jhazmat.2013.12.037
Courbet, C., Rivière, A., Jeannottat, S., Rinaldi, S., Hunkeler, D., Bendjoudi, H., & de Marsily, G. (2011). Complementing approaches to demonstrate chlorinated solvent biodegradation in a complex pollution plume: Mass balance, PCR and compound-specific stable isotope analysis. Journal of Contaminant Hydrology, 126(3), 315–329. https://doi.org/10.1016/j.jconhyd.2011.08.009
DallaLibera, N., Pedretti, D., Tateo, F., Mason, L., Piccinini, L., & Fabbri, P. (2020). Conceptual model of arsenic mobility in the shallow alluvial aquifers near Venice (Italy) elucidated through machine learning and geochemical modeling. Water Resources Research, 56(9), e2019WR026234. https://doi.org/10.1029/2019WR026234
Dalla Libera, N., Pedretti, D., Casiraghi, G., Markó, Á., Piccinini, L., & Fabbri, P. (2021). Probability of non-exceedance of arsenic concentration in groundwater estimated using stochastic multicomponent reactive transport modeling. Water. Multidisciplinary Digital Publishing Institute, 13(21), 3086. https://doi.org/10.3390/w13213086
Davis, J. W., & Carpenter, C. L. (1990). Aerobic biodegradation of vinyl chloride in groundwater samples Applied and Environmental Microbiology. American Society for Microbiology, 56(12), 3878–3880. https://doi.org/10.1128/aem.56.12.3878-3880.1990
Devlin, J. F., & Parker, B. L. (1996). Optimum hydraulic conductivity to limit contaminant flux through cutoff walls. Groundwater, 34(4), 719–726. https://doi.org/10.1111/j.1745-6584.1996.tb02060.x
Dolinová, I., Štrojsová, M., Černík, M., Němeček, J., Macháčková, J., & Ševců, A. (2017). Microbial degradation of chloroethenes: A review. Environmental Science and Pollution Research, 24(15), 13262–13283. https://doi.org/10.1007/s11356-017-8867-y
Edwards, E. A., & Grbić-Galić, D. (1992). Complete mineralization of benzene by aquifer microorganisms under strictly anaerobic conditions Applied and Environmental Microbiology. American Society for Microbiology, 58(8), 2663–2666.
EPA. (2001). Cost analyses for selected groundwater cleanup projects: Pump and treat systems and permeable reactive barriers. Environmental Protection Agency.
Essabri, A. M. A., Aydinlik, N. P., & Williams, N. E. (2019). Bioaugmentation and biostimulation of total petroleum hydrocarbon degradation in a petroleum-contaminated soil with fungi isolated from olive oil effluent. Water, Air, & Soil Pollution, 230(3), 76. https://doi.org/10.1007/s11270-019-4127-8
Fennell, D. E., Carroll, A. B., Gossett, J. M., & Zinder, S. H. (2001). Assessment of indigenous reductive dechlorinating potential at a TCE-contaminated site using microcosms, polymerase chain reaction analysis, and site data. Environmental Science & Technology American Chemical Society, 35(9), 1830–1839. https://doi.org/10.1021/es0016203
Foght, J. (2008). Anaerobic biodegradation of aromatic hydrocarbons: Pathways and prospects. Microbial Physiology. Karger Publishers, 15(2–3), 93–120. https://doi.org/10.1159/000121324
Frascari, D., Zanaroli, G., & Danko, A. S. (2015). In situ aerobic cometabolism of chlorinated solvents: A review. Journal of Hazardous Materials, 283, 382–399. https://doi.org/10.1016/j.jhazmat.2014.09.041
Fritsche, W., Hofrichter, M. (2004). Aerobic degradation of recalcitrant organic compounds by microorganisms. Environmental Biotechnology (pp. 203–227). John Wiley & Sons, Ltd. https://doi-org.pros1.lib.unimi.it/10.1002/3527604286.ch7
Haritash, A. K., & Kaushik, C. P. (2009). Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): A review. Journal of Hazardous Materials, 169(1), 1–15. https://doi.org/10.1016/j.jhazmat.2009.03.137
He, Y. T., Wilson, J. T., Su, C., & Wilkin, R. T. (2015). Review of abiotic degradation of chlorinated solvents by reactive iron minerals in aquifers. Groundwater Monitoring & Remediation, 35(3), 57–75. Wiley Online Library.
Holliger, C., Wohlfarth, G., & Diekert, G. (1998). Reductive dechlorination in the energy metabolism of anaerobic bacteria. FEMS Microbiology Reviews, 22(5), 383–398. https://doi.org/10.1111/j.1574-6976.1998.tb00377.x
Hyun, S. P., & Hayes, K. F. (2009). Feasibility of using in situ FeS precipitation for TCE degradation. Journal of Environmental Engineering American Society of Civil Engineers, 135(10), 1009–1014. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000073
Ismail, N. A., Kasmuri, N., & Hamzah, N. (2022). Microbial bioremediation techniques for polycyclic aromatic hydrocarbon (PAHs)—a review. Water, Air, & Soil Pollution, 233(4), 124. https://doi.org/10.1007/s11270-022-05598-6
Jeong, H. Y., & Hayes, K. F. (2007). Reductive dechlorination of tetrachloroethylene and trichloroethylene by Mackinawite (FeS) in the presence of metals: Reaction rates. Environmental Science & Technology American Chemical Society, 41(18), 6390–6396. https://doi.org/10.1021/es0706394
Kao, C. M., Chen, S. C., Wang, J. Y., Chen, Y. L., & Lee, S. Z. (2003). Remediation of PCE-contaminated aquifer by an in situ two-layer biobarrier: Laboratory batch and column studies. Water Research, 37(1), 27–38. https://doi.org/10.1016/S0043-1354(02)00254-3
Kirk, M. F., Holm, T. R., Park, J., Jin, Q., Sanford, R. A., Fouke, B. W., & Bethke, C. M. (2004). Bacterial sulfate reduction limits natural arsenic contamination in groundwater. Geology, 32(11), 953–956. https://doi.org/10.1130/G20842.1
Kirk, M. F., Roden, E. E., Crossey, L. J., Brealey, A. J., & Spilde, M. N. (2010). Experimental analysis of arsenic precipitation during microbial sulfate and iron reduction in model aquifer sediment reactors. Geochimica Et Cosmochimica Acta, 74(9), 2538–2555. https://doi.org/10.1016/j.gca.2010.02.002
Lang, F. S., Destain, J., Delvigne, F., Druart, P., Ongena, M., & Thonart, P. (2016). Biodegradation of polycyclic aromatic hydrocarbons in mangrove sediments under different strategies: Natural attenuation, biostimulation, and bioaugmentation with Rhodococcus erythropolis T9021. Water, Air, & Soil Pollution, 227(9), 297. https://doi.org/10.1007/s11270-016-2999-4
Lee, M. D., Thomas, J. M., Borden, R. C., Bedient, P. B., Ward, C. H., Wilson, J. T., & Conway, R. A. (1988). Biorestoration of aquifers contaminated with organic compounds. Critical Reviews in Environmental Control, 18(1), 29–89. https://doi.org/10.1080/10643388809388342 Taylor & Francis.
Lendvay, J. M., Löffler, F. E., Dollhopf, M., Aiello, M. R., Daniels, G., Fathepure, B. Z., Gebhard, M., Heine, R., Helton, R., Shi, J., Krajmalnik-Brown, R., Major, C. L., Barcelona, M. J., Petrovskis, E., Hickey, R., Tiedje, J. M., & Adriaens, P. (2003). Bioreactive barriers: a comparison of bioaugmentation and biostimulation for chlorinated solvent remediation. Environmental Science & Technology American Chemical Society, 37(7), 1422–1431. https://doi.org/10.1021/es025985u
Liang, X., Howlett, M. R., Nelson, J. L., Grant, G., Dworatzek, S., Lacrampe-Couloume, G., Zinder, S. H., Edwards, E. A., & Sherwood Lollar, B. (2011). Pathway-dependent isotope fractionation during aerobic and anaerobic degradation of monochlorobenzene and 1,2,4-trichlorobenzene. Environmental Science & Technology American Chemical Society, 45(19), 8321–8327. https://doi.org/10.1021/es201224x
Liu, Y., Ngo, H. H., Guo, W., Sun, J., Wang, D., Peng, L., & Ni, B.-J. (2017). Modeling aerobic biotransformation of vinyl chloride by vinyl chloride-assimilating bacteria, methanotrophs and ethenotrophs. Journal of Hazardous Materials, 332, 97–103. https://doi.org/10.1016/j.jhazmat.2017.03.003
Luptakova, A., Kotulicova, I., Macingova, E., & Jencarova, J. (2013). Bacterial elimination of sulphates from mine waters. Chemical Engineering Transactions, 35, 853–858. https://doi.org/10.3303/CET1335142
Madsen, E. L., Sinclair, J. L., & Ghiorse, W. C. (1991). In situ biodegradation: microbiological patterns in a contaminated aquifer. Science American Association for the Advancement of Science, 252(5007), 830–833.
Majone, M., Verdini, R., Aulenta, F., Rossetti, S., Tandoi, V., Kalogerakis, N., Agathos, S., Puig, S., Zanaroli, G., & Fava, F. (2015). In situ groundwater and sediment bioremediation: Barriers and perspectives at European contaminated sites. New Biotechnology, 32(1), 133–146. https://doi.org/10.1016/j.nbt.2014.02.011
Mattes, T. E., Alexander, A. K., & Coleman, N. V. (2010). Aerobic biodegradation of the chloroethenes: Pathways, enzymes, ecology, and evolution. FEMS Microbiology Reviews, 34(4), 445–475. https://doi.org/10.1111/j.1574-6976.2010.00210.x
Matteucci, F., Ercole, C., & del Gallo, M. (2015). A study of chlorinated solvent contamination of the aquifers of an industrial area in central Italy: A possibility of bioremediation. Frontiers in Microbiology, 6(924), 1–10. https://doi.org/10.3389/fmicb.2015.00924
Moore, J. N., Ficklin, W. H., & Johns, C. (1988). Partitioning of arsenic and metals in reducing sulfidic sediments. Environmental Science & Technology. ACS Publications, 22(4), 432–437.
Mukwevho, M. J., Chirwa, E. M. N., & Maharajh, D. (2019). The effect of pH and temperature on biological sulphate reduction. The Italian Association of Chemical Engineering, 74, 517–522. https://doi.org/10.3303/CET1974087
Nowak, J., Kirsch, N. H., Hegemann, W., & Stan, H.-J. (1996). Total reductive dechlorination of chlorobenzenes to benzene by a methanogenic mixed culture enriched from Saale river sediment. Applied Microbiology and Biotechnology, 45(5), 700–709. https://doi.org/10.1007/s002530050751
O’Day, P. A., Vlassopoulos, D., Root, R., & Rivera, N. (2004). The influence of sulfur and iron on dissolved arsenic concentrations in the shallow subsurface under changing redox conditions. Proceedings of the National Academy of Sciences National Acad Sciences, 101(38), 13703–13708.
Pedretti, D. (2020). Heterogeneity-controlled uncertain optimization of pump-and-treat systems explained through geological entropy. GEM - International Journal on Geomathematics, 11(1), 22. https://doi.org/10.1007/s13137-020-00158-8
Pedretti, D., Fernàndez-Garcia, D., Bolster, D., & Sanchez-Vila, X. (2013). On the formation of breakthrough curves tailing during convergent flow tracer tests in three-dimensional heterogeneous aquifers. Water Resources Research, 49(7), 4157–4173. https://doi.org/10.1002/wrcr.20330
Pi, K., Wang, Y., Xie, X., Ma, T., Liu, Y., Su, C., Zhu, Y., & Wang, Z. (2017). Remediation of arsenic-contaminated groundwater by in-situ stimulating biogenic precipitation of iron sulfides. Water Research, 109, 337–346. https://doi.org/10.1016/j.watres.2016.10.056
Rittle, K. A., Drever, J. I., & Colberg, P. J. (1995). Precipitation of arsenic during bacterial sulfate reduction. Geomicrobiology Journal. Taylor & Francis, 13(1), 1–11.
Singh, J., Knapp, H.V., Demissie, M. (2004). Hydrologic modeling of the Iroquois River watershed using HSPF and SWAT. ISWS CR 2004–08. Champaign, Ill.: Illinois State Water Survey, 41(2), 343–360. https://doi-org.pros1.lib.unimi.it/10.1111/j.1752-1688.2005.tb03740.x
Sturman, P. J., Stewart, P. S., Cunningham, A. B., Bouwer, E. J., & Wolfram, J. H. (1995). Engineering scale-up of in situ bioremediation processes: A review. Journal of Contaminant Hydrology, 19(3), 171–203. https://doi.org/10.1016/0169-7722(95)00017-P
Suthersan, S. S., Schnobrich, M., Martin, J., Horst, J. F., & Gates, E. (2017). Three decades of solvent bioremediation: The evolution from innovation to conventional practice. Groundwater Monitoring & Remediation, 37(2), 14–23. Wiley Online Library.
Thomas, J. M., & Ward, C. H. (1989). In situ biorestoration of organic contaminants in the subsurface. Environmental Science & Technology, 23(7), 760–766. https://doi.org/10.1021/es00065a004
Tiehm, A., & Schmidt, K. R. (2011). Sequential anaerobic/aerobic biodegradation of chloroethenes—aspects of field application. Current Opinion in Biotechnology, 22(3), 415–421. https://doi.org/10.1016/j.copbio.2011.02.003
Tobiszewski, M., & Namieśnik, J. (2012). Abiotic degradation of chlorinated ethanes and ethenes in water. Environmental Science and Pollution Research, 19(6), 1994–2006. https://doi.org/10.1007/s11356-012-0764-9
Vanbroekhoven, K., Van Roy, S., Gielen, C., Maesen, M., Ryngaert, A., Diels, L., & Seuntjens, P. (2007). Microbial processes as key drivers for metal (im)mobilization along a redox gradient in the saturated zone. Environmental Pollution (Barking, Essex: 1987), 148(3), 759–769. https://doi.org/10.1016/j.envpol.2007.01.036
Vogt, C., Kleinsteuber, S., & Richnow, H.-H. (2011). Anaerobic benzene degradation by bacteria. Microbial Biotechnology, 4(6), 710–724. https://doi.org/10.1111/j.1751-7915.2011.00260.x
Acknowledgements
We acknowledge two anonymous Reviewers for their evaluation of our original manuscript.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement. This research was funded by INAIL (Istituto Nazionale per l'Assicurazione contro gli Infortuni sul Lavoro—National Institute for Insurance against Accidents at Work) through the call INAIL-BRIC 2019 (project ID: 52). The results here presented have been developed in the frame of the MIUR Project “Dipartimenti di Eccellenza 2017—Le Geoscienze per la societa’: risorse e loro evoluzione”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Casiraghi, G., Pedretti, D., Beretta, G.P. et al. Piloting Activities for the Design of a Large-scale Biobarrier Involving In Situ Sequential Anaerobic–aerobic Bioremediation of Organochlorides and Hydrocarbons. Water Air Soil Pollut 233, 425 (2022). https://doi.org/10.1007/s11270-022-05886-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05886-1