Abstract
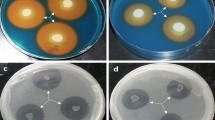
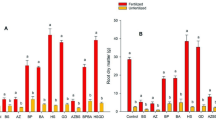
Phytoremediation can be assisted by microorganisms, which promote plant growth and increase heavy metal availability in soil. In this study, we aimed at evaluating the effect of two plant growth-promoting bacteria (PGPB) on phytoextraction of copper (Cu) by maize. We chose the strains based on their ability to synthesize indole compounds, produce siderophores, solubilize phosphorus, and increase soil conductivity and extractable Cu in soil. Then, in glasshouse experiments, we assessed their ability to increase biomass, chlorophyll content, and Cu extraction by maize. Results showed that Acinetobacter sp. RG30 and Pseudomonas putida GN04 were overall the most active strains to synthesize indole, produce siderophores, and solubilize phosphorus, and hence selected for further studies. Also, both were able to significantly increase soil conductivity and release Cu from soil compared to control. Glasshouse experiments showed that Cu had a negative effect on plant growth, but inoculation with bacteria promoted plant growth and chlorophyll content in its presence (p < 0.05). Notably, the effect of inoculation on plant growth was larger on contaminated than on uncontaminated soil, which suggests an overall bacterial effect for alleviation of stress caused by Cu. Inoculation with RG30 or GN04 improved Cu extraction by maize (p < 0.05); interestingly, co-inoculation led to the highest accumulation (200 μg Cu/g plant dry weight). We conclude, therefore, that inoculation with RG30 and GN04 improves metal extraction by increasing plant growth, fitness, and availability of minerals in soil, which represents an important tool for the improvement of phytoextraction processes in polluted environments.




Similar content being viewed by others
References
Abdel-Basset, R., Issa, A. A., & Adam, M. S. (1995). Chlorophyllase activity: effect of heavy metals and calcium. Photosynthetica, 31, 421–425.
Abou-Shanab, R. A., Angle, J. S., Delorme, T. A., Chaney, R. L., van Berkum, P., Moawad, H., Ghanem, K., & Ghozlan, H. A. (2003). Rhizobacterial effects on nickel extraction from soil and uptake by Alyssum murale. New Phytologist, 158, 219–224.
Bashan, Y., Bustillos, J., Leyva, L., Hernandez, J. P., & Bacilio, M. (2006). Increase in auxiliary photoprotective photosynthetic pigments in wheat seedlings induced by Azospirillum brasilense. Soil Biology and Fertility of Soils, 42, 279–285.
Belimov, A. A., Hontzeas, N., Safronova, V. I., Demchinskaya, S. V., Piluzza, G., Bullitta, S., & Glick, B. R. (2005). Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biology and Biochemistry, 37, 241–250.
Bibi, M., & Hussain, M. (2005). Effect of copper and lead on photosynthesis and plant pigments in black gram [Vigna mungo (L.) Hepper]. Bulletin of Environmental and Contamination Toxicology, 74, 1126–1133.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Carreño-Lopez, R., Campos-Reales, N., Elmerich, C., & Baca, B. E. (2000). Physiological evidence for differently regulated tryptophan-dependent pathways for indole-3-acetic acid synthesis in Azospirillum brasilense. Molecular Genetics and Genomics, 264, 521–530.
Cataldo, D. A., & Wildung, R. E. (1978). Soil and plant factors influencing the accumulation of heavy metals by plants. Environmental Health Perspectives, 27, 149–159.
Chen, Y. X., Wang, Y. P., Lin, Q., & Luo, Y. M. (2005). Effect of copper-tolerant rhizosphere bacteria on mobility of copper in soil and copper accumulation by Elsholtzia splendens. Environment International, 31, 861–866.
Drazkiewicz, M., & Baszynski, T. (2005). Growth parameters and photosynthetic pigments in leaf segments of Zea mays exposed to cadmium, as related to protection mechanisms. Journal of Plant Physiology, 162, 1013–1021.
Estrada, G. A., Baldani, V. L. D., Oliveira, D., Urquiaga, S., & Baldani, J. I. (2013). Selection of phosphate-solubilizing diazotrophic Herbaspirillum and Burkholderia strains and their effect on rice crop yield and nutrient uptake. Plant and Soil, 369, 115–129.
Fernandes, J., & Henriques, F. (1991). Biochemical, physiological, and structural effects of excess copper in plants. Botanical Reviews, 57, 246–273.
Fiske, C. H., & Subbarow, Y. (1925). The colorimetric determination of phosphorus. Journal of Biological Chemistry, 66, 375–400.
Flemming, C. A., & Trevors, J. T. (1989). Copper toxicity and chemistry in the environment: a review. Water, Air, and Soil Pollution, 44, 143–158.
Garcia-Rosales, G., & Colin-Cruz, A. (2010). Biosorption of lead by maize (Zea mays) stalk sponge. Journal of Environmental Management, 91, 2079–2086.
Glick, B. R. (2010). Using soil bacteria to facilitate phytoremediation. Biotechnology Advances, 28, 367–374.
Glickmann, E., & Dessaux, Y. (1995). A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Applied and Environmental Microbiology, 61, 793–796.
Gupta, D. K., Srivastava, A., & Singh, V. P. (2008). EDTA enhances lead uptake and facilitates phytoremediation by vetiver grass. Journal of Environmental Biology, 29, 903–906.
Hiscox, J. D., & Israelstam, G. F. (1979). A method for the extraction of chlorophyll from leaf tissue without maceration. Canadian Journal of Botany, 57, 1332–1334.
Hovsepyan, A., & Greipsson, S. (2004). Effect of arbuscular mycorrhizal fungi on phytoextraction by corn (Zea mays) of lead-contaminated soil. International Journal of Phytoremediation, 6, 305–321.
Komarek, M., Vanek, A., Mrnka, L., Sudova, R., Szakova, J., Tejnecky, V., & Chrastny, V. (2010). Potential and drawbacks of EDDS-enhanced phytoextraction of copper from contaminated soils. Environmental Pollution, 158, 2428–2438.
Kos, B., & Leštan, D. (2003). Induced phytoextraction/soil washing of lead using biodegradable chelate and permeable barriers. Environmental Science and Technology, 37, 624–629.
Kumar, K. V., Singh, N., Behl, H. M., & Srivastava, S. (2008). Influence of plant growth promoting bacteria and its mutant on heavy metal toxicity in Brassica juncea grown in fly ash amended soil. Chemosphere, 72, 678–683.
Lambrecht, M., Okon, Y., Vande Broek, A., & Vanderleyden, J. (2000). Indole-3-acetic acid: a reciprocal signalling molecule in bacteria-plant interactions. Trends in Microbiology, 8, 298–300.
Lasat, M. M. (2002). Phytoextraction of toxic metals: a review of biological mechanisms. Journal of Environmental Quality, 31, 109–120.
Lichtenthaler, H. K., Buschmann, C., Döll, M., Fietz, H. J., Bach, T., Kozel, U., Meier, D., & Rahmsdorf, U. (1981). Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynthetic Research, 2, 115–141.
López-Chuken, U., Young, S., & Sánchez-González, M. (2010). The use of chloro-complexation to enhance cadmium uptake by Zea mays and Brassica juncea: testing a “free ion activity model” and implications for phytoremediation. International Journal of Phytoremediation, 12, 680–696.
Ma, Y., Rajkumar, M., & Freitas, H. (2009). Inoculation of plant growth promoting bacterium Achromobacter xylosoxidans strain Ax10 for the improvement of copper phytoextraction by Brassica juncea. Journal of Environmental Management, 90, 831–837.
McKnight, D. M., & Morel, F. M. M. (1979). Release of weak and strong copper-complexing agents by algae. Limnology and Oceanography, 24, 823–837.
McKnight, D. M., & Morel, F. M. M. (1980). Copper complexation by siderophores from filamentous blue-green algae. Limnology and Oceanography, 25, 62–71.
Meers, E., Ruttens, A., Hopgood, M., Lesage, E., & Tack, F. M. (2005). Potential of Brassic rapa, Cannabis sativa, Helianthus annuus and Zea mays for phytoextraction of heavy metals from calcareous dredged sediment derived soils. Chemosphere, 61, 561–572.
Miranda, C. D., & Rojas, R. (2006). Copper accumulation by bacteria and transfer to scallop larvae. Marine Pollution Bulletin, 52, 293–300.
Murakami, M., & Ae, N. (2009). Potential for phytoextraction of copper, lead, and zinc by rice (Oryza sativa L.), soybean (Glycine max [L.] Merr.), and maize (Zea mays L.). Journal of Hazardous Materials, 162, 1185–1192.
Nobel, P. S. (2009). Physicochemical and environmental plant physiology (4th ed.). San Diego: Academic.
Ouzounidou, G., Ciamporov, M., Moustakas, M., & Karataglis, S. (1995). Responses of maize (Zea mays L.) plants to copper stress—I. Growth, mineral content and ultrastructure of roots. Environmental and Experimental Botany, 35, 167–176.
Patten, C. L., & Glick, B. (2002). Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Applied and Environmental Microbiology, 68, 3795–3801.
Perrig, D., Boiero, M. L., Masciarelli, O. A., Penna, C., Ruiz, O. A., Cassan, F. D., & Luna, M. V. (2007). Plant-growth-promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Applied Microbiology and Biotechnology, 75, 1143–1150.
Pikovskaya, R. I. (1948). Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya, 17, 362–370.
Pilon, M., Abdel-Ghany, S. E., Cohu, C. M., Gogolin, K. A., & Ye, H. (2006). Copper cofactor delivery in plant cells. Current Opinion in Plant Biology, 9, 256–263.
Pulford, I. D., & Watson, C. (2003). Phytoremediation of heavy metal-contaminated land by trees—a review. Environment International, 29, 529–540.
Purakayastha, T. J., Viswanath, T., Bhadraray, S., Chhonkar, P. K., Adhikari, P. P., & Suribabu, K. (2008). Phytoextraction of zinc, copper, nickel and lead from a contaminated soil by different species of Brassica. International Journal of Phytoremediation, 10, 61–72.
Rajkumar, M., & Freitas, H. (2008). Influence of metal resistant-plant growth-promoting bacteria on the growth of Ricinus communis in soil contaminated with heavy metals. Chemosphere, 71, 834–842.
Rajkumar, M., Nagendran, R., Lee, K. J., Lee, W. H., & Kim, S. Z. (2006). Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere, 62, 741–748.
Ramamoorthy, S., & Kushner, D. J. (1975). Binding of mercuric and other heavy metal ions by microbial growth media. Microbial Ecology, 2, 162–176.
Rodríguez, H., & Fraga, R. (1999). Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnology Advances, 17, 319–339.
Rojas-Tapias, D. F., Bonilla, R., & Dussán, J. (2012a). Effect of inoculation with plant growth-promoting bacteria on growth and copper uptake by sunflowers. Water, Air, and Soil Pollution, 223, 643–654.
Rojas-Tapias, D., Moreno-Galván, A., Pardo-Díaz, S., Obando, M., Rivera, D., & Bonilla, R. (2012b). Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Applied Soil Ecology, 61, 264–272.
Schwyn, B., & Neilands, J. B. (1987). Universal chemical assay for the detection and determination of siderophores. Analytical Biochemistry, 160, 47–56.
Sheng, X. F., Xia, J. J., Jiang, C. Y., He, L. Y., & Qian, M. (2008). Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environmental Pollution, 156, 1164–1170.
Sheng, X., Sun, L., Huang, Z., He, L., Zhang, W., & Chen, Z. (2012). Promotion of growth and Cu accumulation of bio-energy crop (Zea mays) by bacteria: implications for energy plant biomass production and phytoremediation. Journal of Environmental Management, 103, 58–64.
Tandy, S., Schulin, R., & Nowack, B. (2006). The influence of EDDS on the uptake of heavy metals in hydroponically grown sunflowers. Chemosphere, 62, 1454–1463.
Tanyolaç, D., Ekmekci, Y., & Unalan, S. (2007). Changes in photochemical and antioxidant enzyme activities in maize (Zea mays L.) leaves exposed to excess copper. Chemosphere, 67, 89–98.
Wang, H. Q., Lu, S. J., Li, H., & Yao, Z. H. (2007). EDTA-enhanced phytoremediation of lead contaminated soil by Bidens maximowicziana. Journal of Environmental Sciences (China), 19, 1496–1499.
Wellburn, A. (1994). The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Journal of Plant Physiology, 144, 307–313.
Wójcik, M., & Tukiendorf, A. (2003). Response of wild type of Arabidopsis thaliana to copper stress. Biologia Plantarum, 46, 79–84.
Yang, R., Luo, C., Chen, Y., & Wang, G. (2013). Copper-resistant bacteria enhance plant growth and copper phytoextraction. International Journal of Phytoremediation, 15(6), 573–584.
Zevenhuizen, L. P. T. M., Dolfing, J., Eshuis, E. J., & Scholten-Koerselman, I. J. (1979). Inhibitory effects of copper on bacteria related to the free ion concentration. Microbial Ecology, 5, 139–146.
Zhang, H., Kim, M. S., Sun, Y., Dowd, S. E., Shi, H., & Paré, P. W. (2008). Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Molecular Plant Microbe Interactions, 21, 737–744.
Zhi-xin, N., Sun, L. N., Sun, T. H., Li, Y. S., & Wang, H. (2007). Evaluation of phytoextracting cadmium and lead by sunflower, ricinus, alfalfa and mustard in hydroponic culture. Journal of Environmental Sciences (China), 19, 961–967.
Acknowledgments
The authors thank the Biological Science Faculty of the Universidad de los Andes in Colombia and the program Jóvenes Investigadores e Innovadores “Virginia Gutiérrez de Pineda” of Colciencias for its funding and support. Daniel Rojas-Tapias also expresses thanks to Mr. Andrés Moreno Galván by his collaboration throughout the experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rojas-Tapias, D.F., Bonilla, R. & Dussán, J. Effect of Inoculation and Co-inoculation of Acinetobacter sp. RG30 and Pseudomonas putida GN04 on Growth, Fitness, and Copper Accumulation of Maize (Zea mays). Water Air Soil Pollut 225, 2232 (2014). https://doi.org/10.1007/s11270-014-2232-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-2232-2