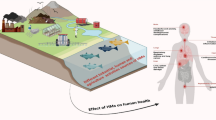
Abstract
A column experiment was conducted to evaluate the effectiveness of nanoscale zerovalent iron (nZVI) for the in situ immobilization of Pb and Zn in an acidic soil. The impact of nZVI on soil was evaluated by monitoring the physicochemical characteristics of the leachates and their ecotoxicological effects on three species, Vibrio fischeri, Artemia franciscana, and Caenorhabditis elegans. Treatment with nZVI resulted in more effective Pb immobilization in comparison to Zn and reduced the leachability by 98 and 72 %, respectively; the immobilization was stable throughout the experiment. Leachates from nZVI-treated soils showed lower toxicity than leachates from untreated ones. The highest toxicity in treated soils was observed in the first leachate, which presented high values of electrical conductivity due to the leachability of soil ions and those provided by the commercial nanoparticle suspension (Na and Fe). V. fischeri and C. elegans were more sensitive to leachates from nZVI-treated soils polluted with Zn than those from soils polluted with Pb; A. franciscana showed the opposite trend.







Similar content being viewed by others
References
Adriano, D. C. (2001). Trace elements in terrestrial environments: biogeochemistry, bioavailability and risk of metals (2nd ed.). Heidelberg: Springer-Verlang New York Berlin.
AFNOR (1991). Détermination de l’inhibition de la luminescence de V. fischeri. NF T90-320, Paris. p. 331.
Anderson, B. S., Hunt, J. W., Phillips, B. M., Fairey, R., Roberts, C. A., Oakden, J. M., et al. (2001). Sediment quality in Los Angeles Harbor, USA: a triad approach. Environmental Toxicology and Chemistry, 20, 359–370.
Boluda, R., Quintanilla, J. F., Bonilla, J. A., Sáez, E., & Gamón, M. (2002). Application of the Microtox® test and pollution indices to the study of water toxicity in the Albufera Natural Park (Valencia, Spain). Chemosphere, 46, 355–369.
Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetic, 77, 71–94.
Chen, S. S., Hsu, H. D., & Li, C. W. (2004). A new method to produce nanoscale iron for nitrate removal. Journal of Nanoparticle Research, 6, 639–647.
Chen, P. J., Su, C. H., Tseng, C. Y., Tan, S. W., & Cheng, C. H. (2011). Toxicity assessments of nanoscale zerovalent iron and its oxidation products in medaka (Oryzias latipes) fish. Marine Pollution Bulletin, 63, 339–346.
Crane, R. A., & Scott, T. B. (2012). Nanoscale zero-valent iron: future prospects for an emerging water treatment technology. Journal of Hazardous Materials, 211–212, 112–125.
Cullen, L. G., Tilston, E. L., Mitchell, G. R., Collins, C. D., & Shaw, L. J. (2011). Assessing the impact of nano- and micro-scale zerovalent iron particles on soil microbial activities: particle reactivity interferes with assay conditions and interpretation of genuine microbial effects. Chemosphere, 82, 1675–1682.
El-Temsah, Y. S., & Joner, E. J. (2012). Ecotoxicological effects on earthworms of fresh and aged nano-sized zero-valent iron (nZVI) in soil. Chemosphere, 89, 76–82.
Fajardo, C., Ortiz, L. T., Rodríguez-Membibre, M. L., Nande, M., Lobo, M. C., & Martín, M. (2012). Assessing the impact of zero-valent iron (ZVI) nanotechnology on soil microbial structure and functionality: A molecular approach. Chemosphere, 86, 802–808.
Froehner, K., Backhaus, T., & Grimme, L. H. (2000). Bioassays with Vibrio fischeri for the assessment of delayed toxicity. Chemosphere, 40, 821–828.
Grieger, K. D., Fjordbøge, A., Hartmann, N. B., Eriksson, E., Bjerg, P. L., & Baun, A. (2010). Environmental benefits and risks of zero-valent iron nanoparticles (nZVI) for in situ remediation: risk mitigation or trade-off? Journal of Contaminant Hydrology, 118, 165–183.
Handy, R. D., van den Brink, N., Chappell, M., Mühling, M., Behra, R., Dušinská, M., et al. (2012). Practical considerations for conducting ecotoxicity test methods with manufactured nanomaterials: what have we learnt so far? Ecotoxicology, 21, 933–972.
Höss, S., Jänsch, S., Moser, T., Junker, T., & Römbke, J. (2009). Assessing the toxicity of contaminated soils using the nematode Caenorhabditis elegans as test organism. Ecotoxicology and Environmental Safety, 72, 1811–1818.
Karn, B., Kuiken, T., & Otto, M. (2009). Nanotechnology and in situ remediation: a review of the benefits and potential risks. Environmental Health Perspectives, 117, 1823–1831.
Keller, A. A., Garner, K., Miller, R. J., & Lenihan, H. S. (2012). Toxicity of nano-zero valent iron to freshwater and marine organisms. PLoS ONE, 7(8), e43983.
Klimkova, S., Cernik, M., Lacinova, L., Filip, J., Jancik, D., & Zboril, R. (2011). Zero-valent iron nanoparticles in treatment of acid mine water from in situ uranium leaching. Chemosphere, 82, 1178–1184.
Kokkali, V., Katramados, I., & Newman, J. D. (2011). Monitoring the effect of metal ions on the mobility of Artemia salina nauplii. Biosensors, 1, 36–45.
Li, X., & Zhang, W. (2007). Sequestration of metal cations with zerovalent iron nanoparticles—a study with high resolution X-ray photoelectron spectroscopy (HR-XPS). Journal of Physical Chemistry C, 111, 6939–6946.
Li, X., Elliott, D. W., & Zhang, W. (2006). Zero-valent iron nanoparticles for abatement of environmental pollutants: materials and engineering aspects. Critical Reviews in Solid State and Materials Sciences, 31, 11–22.
Li, H., Zhou, Q., Wu, Y., Fu, J., Wang, T., & Jiang, G. (2009). Effects of waterborne nano-iron on Medaka (Oryzias latipes): antioxidant enzymatic activity, lipid peroxidation and histopathology. Ecotoxicology and Environmental Safety, 72, 684–692.
Liendo, M. A., Navarro, G. E., & Sampaio, C. H. (2013). Nano and micro ZVI in aqueous media: copper uptake and solution behavior. Water, Air, & Soil Pollution, 224, 1541.
MAPA (1994). Métodos Oficiales de Análisis, vol. III, Spain.
Mortimer, M., Kasemets, K., Heinlaan, M., Kurvet, I., & Kahru, A. (2008). High throughput kinetic Vibrio fischeri bioluminescence inhibition assay for study of toxic effects of nanoparticles. Toxicology in Vitro, 22, 1412–1417.
Mueller, N. C., Braun, J., Bruns, J., Černík, M., Rissing, P., Rickerby, D., et al. (2012). Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe. Environmental Science and Pollution Research, 19, 550–558.
Pawlett, M., Ritz, K., Dorey, R. A., Rocks, S., Ramsden, J., & Harris, J. A. (2013). The impact of zero-valent iron nanoparticles upon soil microbial communities is context dependent. Environmental Science and Pollution Research, 20, 1041–1049.
Persoone, G., Van de Vell, A., Van Steertegem, M., & Nayer, B. (1989). Predictive value for laboratory tests with aquatic invertebrates: influence of experimental conditions. Aquatic Toxicology, 14, 149–166.
Reddy, K. R., Kadlec, R. H., Flaig, E., & Gale, P. M. (1999). Phosphorus retention in streams and wetlands-a review. Critical Reviews in Environmental Science and Technology, 29, 86–146.
Rhue, R. D., & Kamprath, E. J. (1973). Leaching losses of sulfur during winter months when applied as gypsum, elemental S or prilled S. Agronomy Journal, 65, 603–605.
Roh, J. Y., Lee, J., & Choi, J. (2006). Assessment of stress-related gene expression in heavy metal-exposed nematode Caenorhabditis elegans: a potential biomarker for metal-induced toxicity monitoring and environmental risk assessment. Environmental Toxicology & Chemistry, 25, 2946–2956.
Ryden, J. C., McLaughlin, J. R., & Syers, J. K. (1977). Mechanisms of phosphate sorption by soils and hydrous ferric oxide gel. Journal of Soil Science, 28, 72–92.
Sánchez-Fortún, S., Sanz, F., Santa-María, A., Ros, J. M., De Vicente, M. L., Encinas, M. T., et al. (1997). Acute sensitivity of three age classes of Artemia salina larvae to seven chlorinated solvents. Bulletin of Environmental Contamination and Toxicology, 59, 445–451.
Singh, R., Misra, V., & Singh, R. P. (2012). Removal of Cr(VI) by Nanoscale zero-valent iron (nZVI) from soil contaminated with tannery wastes. Bulletin of Environmental Contamination and Toxicology, 88, 210–214.
Terman, G. L. (1977). Quantitative relationships among nutrients leached from soils. Soil Science Society of America Journal, 41, 935–940.
USEPA (1992). Ground Water Issue. Behavior of metals in soils. EPA/540/S-92/018.
van der Oost, R., Beyer, J., & Vermeulen, N. P. E. (2003). Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environmental Toxicology and Pharmacology, 13, 57–149.
Vangronsveld, J., Cunningham, S.D. (1998). Introduction to the concepts. In: Metal-contaminated soils. In situ inactivation and phytorestoration. (eds. J. Vangronsveld , S.D. Cunningham), Springer, New York, pp. 1-15.
Vanhaecke, P., Persoone, G. (1984) The ARC-Test: A standardized short-term routine toxicity test with Artemianauplii. Methodolgy and evaluation. In: G. Persoone, J. Jaspers and C. Claus (Eds.). Ecotoxicological Testing for the Marine Environment. State Univ. of Ghent and Inst. Mar. Scient. Res., Bredene, Belgium vol. 2.
Wright, D. A., & Welbourn, P. (2002). Environmental toxicology. Cambridge, UK: Cambridge University Press.
Xu, Y., & Zhao, D. (2007). Reductive immobilization of chromate in water and soil using stabilized iron nanoparticles. Water Research, 41, 2010–2108.
Zhang, W. (2003). Nanoscale iron particles for environmental remediation: an overview. Journal of Nanoparticle Research, 5, 323–332.
Zhang, M., Wang, Y., Zhao, D., & Pan, G. (2010). Immobilization of arsenic in soils by stabilized nanoscale zero-valent iron, iron sulfide (FeS), and magnetite (Fe3O4) particles. Chinese Science Bulletin, 55, 365–372.
Acknowledgments
The authors thank Ministerio de Educación y Ciencia (Spain) for supporting Project CTM 2010-20617-C02-02 and Consejería de Educación from Comunidad de Madrid for supporting Project S2009/AMB-1478 (EIADES, www.eiades.org).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gil-Díaz, M., Ortiz, L.T., Costa, G. et al. Immobilization and Leaching of Pb and Zn in an Acidic Soil Treated with Zerovalent Iron Nanoparticles (nZVI): Physicochemical and Toxicological Analysis of Leachates. Water Air Soil Pollut 225, 1990 (2014). https://doi.org/10.1007/s11270-014-1990-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-1990-1