Abstract
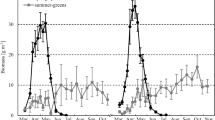
Bryophytes and lichens abound in many arctic ecosystems and can contribute substantially to the ecosystem net primary production (NPP). Because of their growth seasonality and their potential for growth out of the growing season peak, bryophyte and lichen contribution to NPP may be particularly significant when vascular plants are less active and ecosystems act as a source of carbon (C). To clarify these dynamics, nonvascular and vascular aboveground NPP was compared for a subarctic heath during two contrasting periods of the growing season, viz. early-mid summer and late summer-early autumn. Nonvascular NPP was determined by assessing shoot biomass increment of three moss species (Hylocomium splendens, Pleurozium schreberi and Dicranum elongatum) and by scaling to ecosystem level using average standing crop. For D. elongatum, these estimates were compared with production estimates obtained from measurements of shoot length increase. Vascular NPP was determined by harvesting shrub and herb apical growth and considering production due to stem secondary growth of shrubs. Hylocomium splendens and Pleurozium schreberi showed highest biomass growth in late summer, whereas for D. elongatum this occurred in early summer. Maximum relative growth rates were ca. 0.003–0.007 g g−1 d−1. For D. elongatum, production estimates from length growth differed from estimations from biomass growth, likely because of an uncoupling between length growth and biomass shoot growth. Nonvascular NPP was 0.37 and 0.46 g dry weight m−2 d−1, in early and late summer, respectively, whereas in the same periods vascular NPP was 3.6 and 1.1 g dry weight m−2 d−1. The contribution of nonvascular NPP to total aboveground NPP was therefore minor in early summer but substantial in late summer, when 25% of the C accumulated by the vegetation was incorporated into nonvascular plant tissue. The expected global change-induced reduction of nonvascular plant biomass in subarctic heath is likely therefore to enhance C release during the late part of the growing season.



Similar content being viewed by others
References
Asada T, Warner BG (2003) Growth of mosses in relation to climate factors in a hypermaritime coastal peatland in British Columbia, Canada. Bryologist 106:516–527. doi:10.1639/0007-2745(2003)106[516:GOMIRT]2.0.CO;2
Bisbee KE, Gower ST, Norman JM, Nordheim EV (2001) Environmental controls on ground cover species composition and productivity in a boreal black spruce forest. Oecologia 129:261–270. doi:10.1007/s004420100719
Bliss LC (1981) North American and Scandinavian tundras an polar deserts. In: Bliss LC, Heal OW, Moore JJ (eds) Tundra ecosystems: a comparative analysis. Cambridge University Press, Cambridge
Bret-Harte MS, Shaver GR, Chapin FS (2002) Primary and secondary stem growth in arctic shrubs: implications for community response to environmental change. J Ecol 90:251–267. doi:10.1046/j.1365-2745.2001.00657.x
Busby JR, Bliss LC, Hamilton CD (1978) Microclimate control of growth-rates and habitats of boreal forest mosses, Tomenthypnum nitens and Hylocomium splendens. Ecol Monogr 48:95–110. doi:10.2307/2937294
Callaghan TV, Collins NJ, Callaghan CH (1978) Photosynthesis, growth and reproduction of Hylocomium splendens and Polytrichum commune in Swedish Lapland—Strategies of growth and population dynamics of tundra plants. 4. Oikos 31:73–88. doi:10.2307/3543386
Chapin FS, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA (1995) Responses of Arctic tundra to experimental and observed changes in climate. Ecology 76:694–711. doi:10.2307/1939337
Christensen TR, Michelsen A, Jonasson S, Schmidt IK (1997) Carbon dioxide and methane exchange of a subarctic heath in response to climate change related environmental manipulations. Oikos 79:34–44. doi:10.2307/3546087
Clymo RS (1970) The growth of Sphagnum: methods of measurements. J Ecol 58:13–49. doi:10.2307/2258168
Douma JC, van Wijk MT, Lang SI, Shaver GR (2007) The contribution of mosses to the carbon and water exchange of arctic ecosystems: quantification and relationships with system properties. Plant Cell Environ 30:1205–1215. doi:10.1111/j.1365-3040.2007.01697.x
Goulden ML, Crill PM (1997) Automated measurements of CO2 exchange at the moss surface of a black spruce forest. Tree Physiol 17:537–542
Goulden ML, Wofsy SC, Harden JW, Trumbore SE, Crill PM, Gower ST, Fries T, Daube BC, Fan SM, Sutton DJ, Bazzaz A, Munger JW (1998) Sensitivity of boreal forest carbon balance to soil thaw. Science 279:214–217. doi:10.1126/science.279.5348.214
Groendahl L, Friborg T, Soegaard H (2007) Temperature and snow-melt controls on interannual variability in carbon exchange in the high Arctic. Theor Appl Climatol 88:111–125. doi:10.1007/s00704-005-0228-y
Grogan P, Jonasson S (2005) Temperature and substrate controls on intra-annual variation in ecosystem respiration in two subarctic vegetation types. Glob Change Biol 11:465–475. doi:10.1111/j.1365-2486.2005.00912.x
Grogan P, Jonasson S (2006) Ecosystem CO2 production during winter in a Swedish subarctic region: the relative importance of climate and vegetation type. Glob Change Biol 12:1479–1495. doi:10.1111/j.1365-2486.2006.01184.x
Grogan P, Illeris L, Michelsen A, Jonasson S (2001) Respiration of recently-fixed plant carbon dominates mid-winter ecosystem CO2 production in sub-arctic heath tundra. Clim Change 50:129–142. doi:10.1023/A:1010610131277
Hanslin HM (1999) Seasonal dynamics of biomass increase and shoot elongation in five co-occurring boreal forest bryophytes. J Bryol 21:5–15
Heijmans M, Arp WT, Chapin FS (2004) Carbon dioxide and water vapour exchange from understory species in boreal forest. Agric Meteorol 123:135–147. doi:10.1016/j.agrformet.2003.12.006
Hicklenton PR, Oechel WC (1976) Physiological aspects of ecology of Dicranum fuscescens in Subarctic.1. Acclimation and acclimation potential of CO2 exchange in relation to habitat, light, and temperature. Can J Bot 54:1104–1119. doi:10.1139/b76-118
Hobbie SE (1996) Temperature and plant species control over litter decomposition in Alaskan tundra. Ecol Monogr 66:503–522. doi:10.2307/2963492
Hobbie SE, Chapin FS (1998) Response of tundra plant biomass, aboveground production, nitrogen, and CO2 flux to experimental warming. Ecology 79:1526–1544
Illeris L, König SM, Grogan P, Jonasson S, Michelsen A, Ro-Poulsen H (2004) Growing-season carbon dioxide flux in a dry subarctic heath: responses to long-term manipulations. Arct Antarct Alp Res 36:456–463. doi:10.1657/1523-0430(2004)036[0456:GCDFIA]2.0.CO;2
Jonasson S (1982) Organic matter and phytomass on three north Swedish tundra sites, and some connections with adjacent tundra areas. Holarct Ecol 5:367–375
Kallio P, Heinonen S (1975) CO2 exchange and growth of Rhacomitrium lanuginosum and Dicranum elongatum. In: Wielgolaski FE (ed) Fennoscandian tundra ecosystems. Part 1: plants and microorganisms. Springer-Verlag, Berlin
Kjelvik S, Kärenlampi L (1975) Plant biomass and primary production of fennoscandian subarctic and subalpine forests and of alpine willow and heath ecosystems. In: Wielgolaski FE (ed) Fennoscandian tundra ecosystems. Part 1: plants and microorganisms. Springer-Verlag, Berlin
Laurila T, Soegaard H, Lloyd CR, Aurela M, Tuovinen JP, Nordstroem C (2001) Seasonal variations of net CO2 exchange in European Arctic ecosystems. Theor Appl Climatol 70:183–201. doi:10.1007/s007040170014
Lloyd CR (2001) The measurement and modelling of the carbon dioxide exchange at a high Arctic site in Svalbard. Glob Change Biol 7:405–426. doi:10.1046/j.1365-2486.2001.00422.x
Maxwell B (1997) Recent climate patterns in the Arctic. In: Oechel WC, Callaghan TV, Gilmanov T, Holten JI, Maxwell B, Molau U, Sveinbjörnsson B (eds) Global change and Arctic terrestrial ecosystems. Springer-Verlag, New York
Morén AS, Lindroth A (2000) CO2 exchange at the floor of a boreal forest. Agric Meteorol 101:1–14. doi:10.1016/S0168-1923(99)00160-4
Murray KJ, Tenhunen JD, Kummerow J (1989) Limitations on Sphagnum growth and net primary production in the foothills of the Philip Smith Mountains, Alaska. Oecologia 80:256–262
Oechel WC, Vourlitis GL, Hastings SJ, Zulueta RC, Hinzman L, Kane D (2000) Acclimation of ecosystem CO2 exchange in the Alaskan Arctic in response to decadal climate warming. Nature 406:978–981. doi:10.1038/35023137
Post WM, Emanuel WR, Zinke PJ, Stangenberger AG (1982) Soil carbon pools and world life zones. Nature 298:156–159. doi:10.1038/298156a0
Rincon E, Grime JP (1989) An analysis of seasonal patterns of bryophyte growth in a natural habitat. J Ecol 77:447–455. doi:10.2307/2260761
Shaver GR (1986) Woody stem production in Alaskan tundra shrubs. Ecology 67:660–669. doi:10.2307/1937690
Shaver GR, Chapin FS (1991) Production–biomass relationships and element cycling in contrasting arctic vegetation types. Ecol Monogr 61:1–31. doi:10.2307/1942997
Shaver GR, Jonasson S (2001) Productivity of arctic ecosystems. In: Roy J, Saugier B, Mooney HA (eds) Terrestrial global productivity. Academic Press, San Diego
Shaver GR, Laundre JA, Giblin AE, Nadelhoffer KJ (1996) Changes in live plant biomass, primary production and species composition along a riverside toposequence in Arctic Alaska, USA. Arct Alp Res 28:363–379. doi:10.2307/1552116
Sommerkorn M, Bölter M, Kappen L (1999) Carbon dioxide fluxes of soils and mosses in wet tundra of Taimyr Peninsula, Siberia: controlling factors and contribution to net system fluxes. Polar Res 18:253–260. doi:10.1111/j.1751-8369.1999.tb00301.x
Sonesson M (1973) Studies in production and turnover of bryophytes at Stordalen, 1972. In: Sonesson M (ed) Progress Report 1972. Swedish IBP Tundra Project Tech. Rep
Sonesson M, Johansson S (1974) Bryophyte growth, Stordalen 1973. In: Flower-Ellis JGK (ed) Progress report 1973. Technical Report No. 16 of the Swedish IBP Tundra Biome Project. Swedish Natural Science Research Council, Stockholm, pp 17–27
Sonesson M, Carlsson BA, Callaghan TV, Halling S, Björn LO, Bertgren M, Johanson U (2002) Growth of two peat-forming mosses in subarctic mires: species interactions and effects of simulated climate change. Oikos 99:151–160. doi:10.1034/j.1600-0706.2002.990115.x
Street LE, Shaver GR, Williams M, Van Wijk MT (2007) What is the relationship between changes in canopy leaf area and changes in photosynthetic CO2 flux in arctic ecosystems? J Ecol 95:139–150. doi:10.1111/j.1365-2745.2006.01187.x
Sveinbjörnsson B, Oechel WC (1992) Control on growth and productivity of bryophytes: environmental limitations under current and anticipated conditions. In: Bates JW, Farmer AM (eds) Bryophytes and lichens in a changing environment. Clarendon Press, Oxford
Swanson RV, Flanagan LB (2001) Environmental regulation of carbon dioxide exchange at the forest floor in a boreal black spruce ecosystem. Agric Meteorol 108:165–181. doi:10.1016/S0168-1923(01)00243-X
van Wijk MT, Clemmensen KE, Shaver GR, Williams M, Callaghan TV, Chapin FS, Cornelissen JHC, Gough L, Hobbie SE, Jonasson S, Lee JA, Michelsen A, Press MC, Richardson SJ, Rueth H (2004) Long-term ecosystem level experiments at Toolik Lake, Alaska, and at Abisko, Northern Sweden: generalizations and differences in ecosystem and plant type responses to global change. Glob Change Biol 10:105–123. doi:10.1111/j.1365-2486.2003.00719.x
van Wijk MT, Williams M, Shaver GR (2005) Tight coupling between leaf area index and foliage N content in arctic plant communities. Oecologia 142:421–427. doi:10.1007/s00442-004-1733-x
Walker DA, Raynolds MK, Daniëls FJA, Einarsson E, Elvebakk A, Gould WA, Katenin AE, Kholod SS, Markon CJ, Melnikov ES, Moskalenko NG, Talbot SS, Yurtsev BA (2005) The circumpolar arctic vegetation map. J Veg Sci 16:267–282. doi:10.1658/1100-9233(2005)016[0267:TCAVM]2.0.CO;2
Wielgolaski FE (1975) Primary productivity of alpine meadow communities. In: Wielgolaski FE (ed) Fennoscandian tundra ecosystems. Part 1: plants and microorganisms. Springer-Verlag, Berlin
Wielgolaski FE, Kärenlampi L (1975) Plant phenology of fennoscandian tundra areas. In: Wielgolaski FE (ed) Fennoscandian tundra ecosystems. Part 1: plants and microorganisms. Springer-Verlag, Berlin
Wielgolaski FE, Bliss LC, Svoboda J, Doyle G (1981) Primary production of tundra. In: Bliss LC, Heal OW, Moore JJ (eds) Tundra ecosystems: a comparative analysis. Cambridge University Press, Cambridge
Williams TG, Flanagan LB (1998) Measuring and modelling environmental influences on photosynthetic gas exchange in Sphagnum and Pleurozium. Plant Cell Environ 21:555–564. doi:10.1046/j.1365-3040.1998.00292.x
Williams M, Eugster W, Rastetter EB, McFadden JP, Chapin FS (2000) The controls on net ecosystem productivity along an Arctic transect: a model comparison with flux measurements. Glob Change Biol 6:116–126. doi:10.1046/j.1365-2486.2000.06016.x
Acknowledgements
This work was funded by the Special Research Fund (BOF) of Ghent University through a PhD grant to the first author (011D07004) and by the EU ATANS Grant (Fp6 506004). We thank Thomas Maere and Philipp Theuring for field and laboratory assistance, Annemie Vermeulen for determination of stem secondary growth, Simone Lang for help with moss identification and anonymous referees for valuable comments on previous versions of the manuscript. Special thanks are due to the Abisko Scientific Research Station for the logistic support and for provision of the meteorological data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Campioli, M., Samson, R., Michelsen, A. et al. Nonvascular contribution to ecosystem NPP in a subarctic heath during early and late growing season. Plant Ecol 202, 41–53 (2009). https://doi.org/10.1007/s11258-008-9527-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-008-9527-6