Abstract
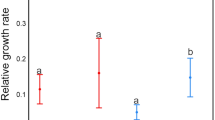
The reserve ovary model is a key hypothesis proposed to explain why plants produce surplus flowers and posits that plants may utilize surplus flowers to compensate for losses from floral herbivory. We tested this hypothesis in the prairie plant Eryngium yuccifolium and its floral herbivore Coleotechnites eryngiella. At five Illinois tallgrass prairie sites, we collected central, primary lateral, and secondary lateral inflorescences from E. yuccifolium to determine whether damage by the larvae of C. eryngiella to the flowers in earlier developing inflorescences would be compensated for in later developing inflorescences. Coleotechnites eryngiella does extensive damage to the central and primary inflorescences and little damage to the secondary inflorescences. Later maturing inflorescences did not compensate for early damage by increasing seed production in later inflorescences. The secondary inflorescences of E. yuccifolium may only compensate for catastrophic damage done to the central and primary inflorescences early on in development, serve as additional advertisements for pollinators, act as pollen donors, or allow the plant to take advantage of “ecological windows” of high pollinator and low herbivore abundance. Our findings were spatially and temporally consistent and did not support the predictions of the reserve ovary model in the E. yuccifolium–C. eryngiella system suggesting that in this system, alternate, proximate, and ultimate causes need to be explored for the production of surplus flowers.



Similar content being viewed by others
References
Arbuckle JL (2006) Amos 7.0 user’s guide. Amos Development Corporation, Spring House, PA, 583 pp
Augspurger CK (1980) Mass-flowering of a tropical shrub (Hybanthus prunifolius): influence on pollinator attraction and movement. Evolution 34:475–488
Bawa KS, Webb CJ (1984) Flower, fruit and seed abortion in tropical rain forest trees: implications for the evolution of paternal and maternal reproductive patterns. Am J Bot 71:736–751
Bertin RI (1990) Effects of pollination intensity in Campsis radicans. Am J Bot 77:178–187
Burnham KP, Anderson DR (1998) Model selection and inference. Springer-Verlag, New York, pp 353
Danderson CA (2006) Notes on aspects of the life history and behavior of Coleotechnites eryngiella (Gelechiidae). J Lepid Soc 60:103–106
Diggle PK (1995) Architectural effects and the interpretation of patterns of fruit and seed development. Annu Rev Ecol Syst 26:531–552
Ehrlén J (1991) Why do plants produce surplus flowers? A reserve-ovary model. Am Nat 138:918–933
Gaudeil M, Till-Bottraud I (2004) Reproductive ecology of the endangered alpine species Eryngium alpinum L. (Apiaceae): phenology, gene dispersal and reproductive success. Ann Bot 93:711–721
Guitian J (1993) Why Prunus mahaleb (Rosaceae) produces more flowers than fruit. Am J Bot 80:1305–1309
Hendrix SD (1979) Compensatory reproduction in a biennial herb following insect defloration. Oecologia 42:107–118
Hendrix SD (1984) Reactions of Heracleum lanatum to floral herbivory by Depressaria pastinacella. Ecology 65:191–197
Hendrix SD, Trapp EJ (1989) Floral herbivory in Pastinaca sativa: do compensatory responses offset reductions in fitness? Evolution 43:891–895
Holtsford TP (1985) Nonfruiting hermaphroditic flowers of Calochortus leichtlinii (Liliaceae): potential reproductive functions. Am J Bot 72:1687–1694
Inouye DW (1982) The consequences of herbivory: a mixed blessing for Jurinea mollis (Asteraceae). Oikos 39:269–272
Iverson LR, Ketzner D, Karnes J (1999) Illinois Plant Information Network, http://www.fs.fed.us/ne/delaware/ilpin/ilpin.html, Cited 16 Dec 2003
Janzen DH (1977) A note on optimal mate selection by plants. Am Nat 111:365–371
Kliber A, Eckert CG (2004) Sequential decline in allocation among flowers within inflorescneces: proximate mechanisms and adaptive significance. Ecology 85:1675–1687
Linville SU (1995) Resource allocation in sequentially flowering Cosmos bipinnatus. Am Midl Nat 134:84–89
Lowenberg GJ (1994) Effects of floral herbivory on maternal reproduction in Sanicula arctopoides (Apiaceae). Ecology 75:359–369
Maschinski J, Whitham TG (1989) The continuum of plant responses to herbivory: the influence of plant association, nutrient availability, and timing. Am Midl Nat 134:1–19
Molano-Flores B (2001) Reproductive biology of Eryngium yuccifolium (Apiaceae), a prairie species. J Torrey Bot Soc 128:1–6
Paige KN (1992) Overcompensation in response to mammalian herbivory: from mutualistic to antagonistic interactions. Ecology 73:2076–2085
Stephenson AG (1980) Fruit set, herbivory, fruit reduction and the fruiting strategy of Catalpa speciosa (Bignoniaceae). Ecology 61:57–64
Stephenson AG (1981) Flower and fruit abortion: proximate causes and ultimate functions. Annu Rev Ecol Syst 12:253–279
The PLANTS Database Version 3.5 (2005) USDA, NRCS National Plant Data Center, Baton Rouge, http://plants.usda.gov, Cited 14 Mar 2005
Willson MF, Price PW (1977) The evolution of inflorescence size in Asclepias (Asclepiadaceae). Evolution 31:495–511
Willson MF, Rathcke BJ (1974) Adaptive design of the floral display in Asclepias syriaca L. Am Midl Nat 92:47–57
Wyatt R (1980) The reproductive biology of Asclepias tuberosa I. Flower number, arrangement, and fruit-set. New Phytol 85:119–131
Wyatt R (1982) Inflorescence architecture: how flower number, arrangement, and phenology affect pollination and fruit-set. Am J Bot 69:585–594
Acknowledgments
We thank the Illinois Nature Preserves Commission for permission to use prairie sites for this study. This work was supported in part by the Illinois Natural History Survey and a 2004 Summer Fellowship from the Dept. of Plant Biology at the University of Illinois (Urbana-Champaign) to CAD. Comments from K. Robertson, S. Downie, M. Berenbaum, D. Moore, J. Taft, C. Ivey, and J. Foster helped improve this study and this manuscript. We thank A. Davis for discussions on path analyses used in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Danderson, C.A., Molano-Flores, B. & Raghu, S. Impact of floral herbivory by Coleotechnites eryngiella Bottimer (Lepidoptera: Gelechiidae) on the reproductive output of Eryngium yuccifolium Michaux (Apiaceae): a test of the reserve ovary model. Plant Ecol 197, 277–284 (2008). https://doi.org/10.1007/s11258-007-9377-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-007-9377-7