Abstract
Introduction and hypothesis
Anticholinergics have been established for their efficacy and safety in adults with idiopathic overactive bladder syndrome (OAB-s) but not in children and adolescents. This study was aims to investigate the efficacy and safety of anticholinergics in children and adolescents with idiopathic OAB-s.
Method
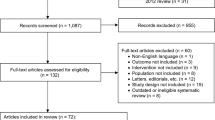
A total of nine studies with 11 trials comprising of 1801 subjects (1116 experimental and 685 controls) were included. Inclusion criteria were idiopathic OAB-s in children or adolescents. Overall SMD of change in diurnal urge incontinence per week, change in mean voiding frequency per 24 h, change in mean voided volume, and incidence of adverse events compared with placebo were investigated.
Results
Overall SMD of diurnal urge incontinence per week for the anticholinergic group (experimental group) vs. the placebo group (control group) was − 0.15 (95% CI − 0.31, 0.01). Overall SMD of mean voiding frequency per 24 h was − 0.16 (95% CI − 0.33, 0.02). Overall SMD of mean voided volume was 0.49 (95% CI 0.10, 0.88). The overall incidence of any AEs of anticholinergics compared with placebo was OR = 1.06 (95% CI 0.84–1.34) (p = 0.637). Among each AEs, the only incidence of urinary tract infection showed a higher incidence rate for anticholinergics (OR = 1.92, 95% CI 1.06–3.49) than for placebo.
Conclusions
Apart from oxybutynin, other anticholinergics showed efficacy including an increase in mean voided volume. Moreover, there was no significant difference in the incidence of overall adverse events between anticholinergics and placebo.


Similar content being viewed by others
References
Franco I (2016) Overactive bladder in children. Nat Rev Urol 13(9):520–532. https://doi.org/10.1038/nrurol.2016.152
Kalo BB, Bella H (1996) Enuresis: prevalence and associated factors among primary school children in Saudi Arabia. Acta Paediatr 85(10):1217–1222
Butler RJ, Golding J, Northstone K, Team AS (2005) Nocturnal enuresis at 7.5 years old: prevalence and analysis of clinical signs. BJU Int 96(3):404–410. https://doi.org/10.1111/j.1464-410x.2005.05640.x
Bakker E, van Sprundel M, van der Auwera JC, van Gool JD, Wyndaele JJ (2002) Voiding habits and wetting in a population of 4,332 Belgian schoolchildren aged between 10 and 14 years. Scand J Urol Nephrol 36(5):354–362. https://doi.org/10.1080/003655902320783863
Fitzgerald MP, Thom DH, Wassel-Fyr C, Subak L, Brubaker L, Van Den Eeden SK, Brown JS, Reproductive Risks for Incontinence Study at Kaiser Research G (2006) Childhood urinary symptoms predict adult overactive bladder symptoms. J Urol 175(3 Pt 1):989–993. https://doi.org/10.1016/s0022-5347(05)00416-7
Blais AS, Bergeron M, Nadeau G, Ramsay S, Bolduc S (2016) Anticholinergic use in children: persistence and patterns of therapy. Can Urol Assoc J 10(3–4):137–140. https://doi.org/10.5489/cuaj.3527
Schroder A, Thuroff JW (2010) New strategies for medical management of overactive bladder in children. Curr Opin Urol 20(4):313–317. https://doi.org/10.1097/MOU.0b013e32833aa185
Jackson D, Law M, Barrett JK, Turner R, Higgins JP, Salanti G, White IR (2016) Extending DerSimonian and Laird’s methodology to perform network meta-analyses with random inconsistency effects. Stat Med 35(6):819–839. https://doi.org/10.1002/sim.6752
Marschall-Kehrel D, Feustel C, Persson de Geeter C, Stehr M, Radmayr C, Sillen U, Strugala G (2009) Treatment with propiverine in children suffering from nonneurogenic overactive bladder and urinary incontinence: results of a randomized placebo-controlled phase 3 clinical trial. Eur Urol 55(3):729–736. https://doi.org/10.1016/j.eururo.2008.04.062
Birder L, de Groat W, Mills I, Morrison J, Thor K, Drake M (2010) Neural control of the lower urinary tract: peripheral and spinal mechanisms. Neurourol Urodyn 29(1):128–139. https://doi.org/10.1002/nau.20837
Michel MC, Chapple CR (2009) Basic mechanisms of urgency: roles and benefits of pharmacotherapy. World J Urol 27(6):705–709. https://doi.org/10.1007/s00345-009-0446-5
European Medicines Agency (2013) Clinical investigation of medicinal products in the treatment of urinary incontinence. European Medicine Agency. https://www.ema.europa.eu/en/clinical-investigation-medicinal-products-treatment-urinary-incontinence. Accessed 30 May 2019
Lee SD, Chung JM, Kang DI, Ryu DS, Cho WY, Park S (2017) Efficacy and tolerability of solifenacin 5 mg fixed dose in korean children with newly diagnosed idiopathic overactive bladder: a multicenter prospective study. J Korean Med Sci 32(2):329–334. https://doi.org/10.3346/jkms.2017.32.2.329
Newgreen D, Bosman B, Hollestein-Havelaar A, Dahler E, Besuyen R, Sawyer W, Bolduc S, Rittig S (2017) Solifenacin in children and adolescents with overactive bladder: results of a phase 3 randomised clinical trial. Eur Urol 71(3):483–490. https://doi.org/10.1016/j.eururo.2016.08.061
Newgreen D, Bosman B, Hollestein-Havelaar A, Dahler E, Besuyen R, Snijder R, Sawyer W, Rittig S, Bolduc S (2017) Long-term safety and efficacy of solifenacin in children and adolescents with overactive bladder. J Urol 198(4):928–936. https://doi.org/10.1016/j.juro.2017.05.038
Bolduc S, Moore K, Nadeau G, Lebel S, Lamontagne P, Hamel M (2010) Prospective open label study of solifenacin for overactive bladder in children. J Urol 184(4 Suppl):1668–1673. https://doi.org/10.1016/j.juro.2010.03.124
Hoebeke P, De Pooter J, De Caestecker K, Raes A, Dehoorne J, Van Laecke E, Vande Walle J (2009) Solifenacin for therapy resistant overactive bladder. J Urol 182(4 Suppl):2040–2044. https://doi.org/10.1016/j.juro.2009.05.100
Nadeau G, Schroder A, Moore K, Genois L, Lamontagne P, Hamel M, Pellerin E, Bolduc S (2014) Long-term use of solifenacin in pediatric patients with overactive bladder: extension of a prospective open-label study. Can Urol Assoc J 8(3–4):118–123. https://doi.org/10.5489/cuaj.1356
Andersson KE (2011) Antimuscarinic mechanisms and the overactive detrusor: an update. Eur Urol 59(3):377–386. https://doi.org/10.1016/j.eururo.2010.11.040
Finney SM, Andersson KE, Gillespie JI, Stewart LH (2006) Antimuscarinic drugs in detrusor overactivity and the overactive bladder syndrome: motor or sensory actions? BJU Int 98(3):503–507. https://doi.org/10.1111/j.1464-410X.2006.06258.x
Ferrara P, D’Aleo CM, Tarquini E, Salvatore S, Salvaggio E (2001) Side-effects of oral or intravesical oxybutynin chloride in children with spina bifida. BJU Int 87(7):674–678
Giramonti KM, Kogan BA, Halpern LF (2008) The effects of anticholinergic drugs on attention span and short-term memory skills in children. Neurourol Urodyn 27(4):315–318. https://doi.org/10.1002/nau.20507
Sommer BR, O’Hara R, Askari N, Kraemer HC, Kennedy WA 2nd (2005) The effect of oxybutynin treatment on cognition in children with diurnal incontinence. J Urol 173(6):2125–2127. https://doi.org/10.1097/01.ju.0000157685.83573.79
Lopez Pereira P, Miguelez C, Caffarati J, Estornell F, Anguera A (2003) Trospium chloride for the treatment of detrusor instability in children. J Urol 170(5):1978–1981. https://doi.org/10.1097/01.ju.0000085667.05190.ad
Nijman RJ, Borgstein NG, Ellsworth P, Djurhuus JC (2005) Tolterodine treatment for children with symptoms of urinary urge incontinence suggestive of detrusor overactivity: results from 2 randomized, placebo controlled trials. J Urol 173(4):1334–1339. https://doi.org/10.1097/01.ju.0000152322.17542.63
Triantafyllidis A, Charalambous S, Papatsoris AG, Papathanasiou A, Kalaitzis C, Rombis V, Touloupidis S (2005) Management of nocturnal enuresis in Greek children. Pediatr Nephrol 20(9):1343–1345. https://doi.org/10.1007/s00467-005-1921-x
Nijman RJ, Borgstein NG, Ellsworth P, Siggaard C (2007) Long-term tolerability of tolterodine extended release in children 5-11 years of age: results from a 12-month, open-label study. Eur Urol 52(5):1511–1516. https://doi.org/10.1016/j.eururo.2007.05.002
Neveus T, Tullus K (2008) Tolterodine and imipramine in refractory enuresis; a placebo-controlled crossover study. Pediatr Nephrol 23(2):263–267. https://doi.org/10.1007/s00467-007-0662-4
Deng YJ, Ma G, Guo YF, Ge Z, Lu RG, Wang LX, Zhu HB, Chen CJ (2011) Comparisons of efficacy and safety of tolterodine and oxybutynin in children with idiopathic overactive bladder. Zhongguo Dang Dai Er Ke Za Zhi 13(1):26–28
Quintiliano F, Veiga ML, Moraes M, Cunha C, de Oliveira LF, Lordelo P, Bastos Netto JM, Barroso Junior U (2015) Transcutaneous parasacral electrical stimulation vs oxybutynin for the treatment of overactive bladder in children: a randomized clinical trial. J Urol 193(5 Suppl):1749–1753. https://doi.org/10.1016/j.juro.2014.12.001
Funding
This work was supported by Soonchunhyang University Research Fund (Grant no. 2019-0006).
Author information
Authors and Affiliations
Contributions
JH Kim had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: JH Kim. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: JW Noh and JH Kim. Statistical analysis: JW Noh, B Lee, and JH Kim.
Corresponding author
Ethics declarations
Conflict of interest
All authors have completed and submitted the ICMJE Form for disclosure of potential conflicts of interest and none were reported.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11255_2019_2209_MOESM1_ESM.tif
Supplementary Figure 1. Sensitivity analysis of anticholinergics for urge incontinence episode (A), mean voiding frequency (B), and mean voided volume (C) (TIFF 132 kb)
11255_2019_2209_MOESM2_ESM.tif
Supplementary Figure 2. Cumulative analysis of anticholinergics for mean voiding frequency (A) and mean voided volume (B) (TIFF 163 kb)
Rights and permissions
About this article
Cite this article
Noh, JW., Lee, B. & Kim, J.H. Efficacy and safety of anticholinergics for children or adolescents with idiopathic overactive bladder: systematic review and meta-analysis. Int Urol Nephrol 51, 1459–1471 (2019). https://doi.org/10.1007/s11255-019-02209-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-019-02209-y