Abstract
Background
Tissue culture studies found that renal epithelial cells suffer oxidative injury on exposure to high levels of oxalate (Ox) and calcium oxalate (CaOx) crystals; nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, a major source of reactive oxygen species (ROS) production in kidney, has been shown to be involved in this event. The present study aimed to investigate whether this in vitro feature of NADPH oxidase could be confirmed in vivo.
Methods
Animal model of nephrolithiasis was established in adult male Sprague-Dawley rats by administration of 0.8% ethylene glycol (EG) in drinking water for 4 weeks. Simultaneous treatment with apocynin (0.2 g kg−1 day−1) or losartan (30 mg kg−1 day−1) by intragastric administration was performed in rats. At the end of the study, urinary 8-IP, a product of lipid peroxidation, and enzymatic activity of superoxide dismutase (SOD) in kidney homogenates were assessed as markers for state of renal oxidative stress (OS). Expression of NADPH oxidase subunit p47phox in kidney was localized and evaluated by immunohistochemistry, real-time polymerase chain reaction (PCR), and Western blotting. The concentration of angiotensin II in kidney homogenates was determined using radioimmunoassay method.
Results
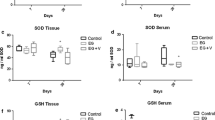
Compared with control, OS developed significantly in rats received EG, with increased expression of p47phox messenger RNA (mRNA) and protein in kidneys. Renal angiotensin II also increased significantly. Treatment with apocynin or losartan significantly reduced excretion of urinary 8-IP, restored SOD activity, with decrease in expression of p47phox in kidney, but levels of those OS markers in apocynin- or losartan-treated rats were still higher than in normal controls.
Conclusions
These results suggest that renal Ang II and its stimulation of NADPH oxidase may partially account for the development of OS in kidney in this rat model of CaOx nephrolithiasis.





Similar content being viewed by others
References
Asplin JR (2002) Hyperoxaluric calcium nephrolithiasis. Endocrinol Metab Clin North Am 31:927–949. doi:10.1016/S0889-8529(02)00030-0
Huang H-S, Ma M-C, Chen J, Chen C-F (2002) Changes in the oxidant–antioxidant balance in the kidneys of rats with nephrolithiasis induced by ethylene glycol. J Urol 167:2584–2593. doi:10.1016/S0022-5347(05)65042-2
Thamilselvan S, Hackett RL, Khan SR (1997) Lipid peroxidation in ethylene glycol induced hyperoxaluria and CaOx nephrolithiasis. J Urol 157:1059–1063. doi:10.1016/S0022-5347(01)65141-3
Huang H-S, Ma M-C, Chen C-F, Chen J (2003) Lipid peroxidation and its correlations with urinary levels of oxalate, citric acid, and osteopontin in patients with renal calcium oxalate stones. Urology 61:1123–1128. doi:10.1016/S0090-4295(03)00764-7
Tungsanga K, Sriboonlue P, Futrakul P, Yachantha C, Tosukhowong P (2005) Renal tubular cell damage and oxidative stress in renal stone patients and the effect of potassium citrate treatment. Urol Res 33:65–69. doi:10.1007/s00240-004-0444-4
Chen DH, Kaung HL, Miller CM, Resnick MI, Marengo SR (2004) Microarray analysis of changes in renal phenotype in the ethylene glycol rat model of urolithiasis: potential and pitfalls. BJU Int 94:637–650. doi:10.1111/j.1464-410X.2004.05016.x
Green ML, Freel RW, Hatch M (2005) Lipid peroxidation is not the underlying cause of renal injury in hyperoxaluric rats. Kidney Int 68:2629–2638. doi:10.1111/j.1523-1755.2005.00735.x
Khan SR (2005) Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res 33:349–357. doi:10.1007/s00240-005-0492-4
Thamilselvan S, Menon M (2005) Vitamin E therapy prevents hyperoxaluria-induced calcium oxalate crystal deposition in the kidney by improving renal tissue antioxidant status. BJU Int 96:117–126. doi:10.1111/j.1464-410X.2005.05579.x
Umekawa T, Byer K, Uemura H, Khan SR (2005) Diphenyleneiodium (DPI) reduces oxalate ion- and calcium oxalate monohydrate and brushite crystal-induced upregulation of MCP-1 in NRK 52E cells. Nephrol Dial Transplant 20:870–878. doi:10.1093/ndt/gfh750
Itoh Y, Yasui T, Okada A, Tozawa K, Hayashi Y, Kohri K (2005) Preventive effects of green tea on renal stone formation and the role of oxidative stress in nephrolithiasis. J Urol 173:271–275
Thamilselvan S, Byer KJ, Hackett RL, Khan SR (2000) Free radical scavengers, catalase and superoxide dismutase provide protection from oxalate-associated injury to LLC-PK1 and MDCK cells. J Urol 164:230–236. doi:10.1016/S0022-5347(05)67499-X
Thamilselvan S, Khan SR, Menon M (2003) Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res 31:3–9
Selvam R (2002) Calcium oxalate stone disease: role of lipid peroxidation and antioxidants. Urol Res 30:35–49. doi:10.1007/s00240-001-0228-z
Geiszt M, Kopp JB, Varnai P, Leto TL (2000) Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA 97:8010–8014. doi:10.1073/pnas.130135897
Hanna IR, Taniyama Y, Szocs K, Rocic P, Griendling KK (2002) NAD(P)H oxidase derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal 4:899–914. doi:10.1089/152308602762197443
Griendling KK, Sorescu D, Ushio-Fukai M (2000) NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86:494–501
Babior BM, Lambeth JD, Nauseef W (2002) The neutrophil NADPH oxidase. Arch Biochem Biophys 397:342–344. doi:10.1006/abbi.2001.2642
Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, Fujita T, Wilcox CS (2005) Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int 67:1890–1898. doi:10.1111/j.1523-1755.2005.00287.x
Paliege A, Parsumathy A, Mizel D, Yang T, Schnermann J, Bachmann S (2006) Effect of apocynin treatment on renal expression of COX-2, NOS1, and renin in Wistar-Kyoto and spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 290:R694–R700. doi:10.1152/ajpregu.00219.2005
Rashed T, Menon M, Thamilselvan S (2004) Molecular mechanism of oxalate-induced free radical production and glutathione redox imbalance in renal epithelial cells: effect of antioxidants. Am J Nephrol 24:557–568. doi:10.1159/000082043
Umekawa T, Hatanaka YJ, Kurita T, Khan SR (2004) Effect of angiotensin II receptor blockage on osteopontin expression and calcium oxalate crystal deposition in rat kidneys. J Am Soc Nephrol 15:635–644. doi:10.1097/01.ASN.0000113321.49771.2D
Toblli JE, Ferder L, Stella I, Angerosa M, Inserra F (2001) Protective role of enalapril for chronic tubulointerstitial lesions of hyperoxaluria. J Urol 166:275–280. doi:10.1016/S0022-5347(05)66144-7
Toblli JE, Ferder L, Stella I, De Cavanagh MVE, Angerosa M, Inserra F (2002) Effects of angiotensin II subtype 1 receptor blockade by losartan on tubulointerstitial lesions caused by hyperoxaluria. J Urol 168:1550–1555. doi:10.1016/S0022-5347(05)64519-3
Khand FD, Gordge MP, Robertson WG, Noronha-Dutra AA, Hothersall JS (2002) Mitochondrial superoxide production during oxalate mediated oxidative stress in renal epithelial cells. Free Radic Biol Med 32:1339–1350. doi:10.1016/S0891-5849(02)00846-8
Cao L-C, Honeyman TW, Cooney R, Kennington L, Scheid CR, Jonassen JA (2004) Mitochondrial dysfunction is a primary event in renal cell oxalate toxicity. Kidney Int 66:1890–1900. doi:10.1111/j.1523-1755.2004.00963.x
Engels F, Renirie BF, Hart BA, Labadie RP, Nijkamp FP (1992) Effects of apocynin, a drug isolated from the roots of Picrorhiza kurroa, on arachidonic acid metabolism. FEBS Lett 305:254–256. doi:10.1016/0014-5793(92)80680-F
Aihara K, Byer KJ, Khan SR (2003) Calcium phosphate-induced renal epithelial injury and stone formation: involvement of reactive oxygen species. Kidney Int 64:1283–1291. doi:10.1046/j.1523-1755.2003.00226.x
Onozato ML, Tojo A, Goto A, Fujita T, Wilcox CS (2002) Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: Effects of ACEI and ARB. Kidney Int 61:186–194. doi:10.1046/j.1523-1755.2002.00123.x
Morrow JD, Roberts LJ (1997) The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res 36:1–21. doi:10.1016/S0163-7827(97)00001-5
Khan SR, Glenton PA, Byer KJ (2006) Modeling of hyperoxaluric calcium oxalate nephrolithiasis: experimental induction of hyperoxaluria by hydroxyl-L-proline. Kidney Int 165:1173–1181
Ueda N, Kaushal GP, Shah SV (2000) Apoptotic mechanisms in acute renal failure. Am J Med 108:403–415. doi:10.1016/S0002-9343(00)00311-9
Gill PS, Wilcox CS (2006) NADPH oxidases in the kidney. Antioxid Redox Signal 8:1597–1607. doi:10.1089/ars.2006.8.1597
Shiose A, Kuroda J, Tsuruya K (2001) A novel superoxide producing NADPH oxidase in kidney. J Biol Chem 276:1417–1423. doi:10.1074/jbc.M007597200
Chabrashvili T, Tojo A, Onozato ML, Kitiyakara C, Quinn MT, Fujita T, Welch WJ, Wilcox CS (2002) Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension 39:269–274. doi:10.1161/hy0202.103264
Li JM, Shah AM (2003) ROS generation by nonphagocytic NADPH oxidase: potential relevance in diabetic nephropathy. J Am Soc Nephrol 14:S221–S226. doi:10.1097/01.ASN.0000077406.67663.E7
Yasunari K, Maeda K, Nakamura M, Yoshikawa J (2002) Pressure promotes angiotensin II-mediated migration of human coronary smooth muscle cells through increase in oxidative stress. Hypertension 39:433–437. doi:10.1161/hy02t2.102991
Lavigne MC, Malech HL, Holland SM, Leto TL (2001) Genetic demonstration of p47phox-dependent superoxide anion production in murine vascular smooth muscle cells. Circulation 104:79–84. doi:10.1161/hc4001.096828
Seikaly MG, Aarnt BS Jr, Seney FD Jr (1990) Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J Clin Invest 86:1352–1357. doi:10.1172/JCI114846
Antus B, Exton MS, Rosivall L (2001) Angiotensin II. A regulator of inflammation during renal disease? Int J Immunopathol Pharmacol 14:25–30
Tojo A, Onozato ML, Kobayashi N, Goto A, Matsuoka H, Fujita T (2002) Angiotensin II and oxidative stress in Dahl Salt-sensitive rat with heart failure. Hypertension 40:834–839. doi:10.1161/01.HYP.0000039506.43589.D5
Acknowledgements
This study was supported by the Major Program of Guangxi Zhuang Autonomous Region Bureau of Health (No. 200729), the Guangxi Science and Technology Development Program (No. 0816004-4), and the Postgraduate Innovation Program (No. 309083).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Cy., Deng, Yl. & Sun, Bh. Effects of apocynin and losartan treatment on renal oxidative stress in a rat model of calcium oxalate nephrolithiasis. Int Urol Nephrol 41, 823–833 (2009). https://doi.org/10.1007/s11255-009-9534-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-009-9534-0