Abstract
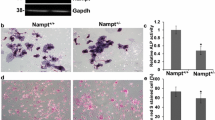
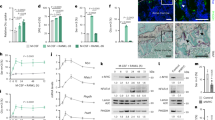
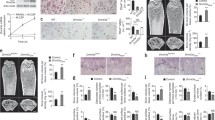
Spermidine/spermine N 1-acetyltransferase (SSAT) is a catabolic regulator of polyamines, ubiquitous molecules essential for cell proliferation and differentiation. In pathological conditions, the increased polyamine catabolism has been shown to mediate its cellular functions not only by changed polyamine levels but also by the availability of metabolites shared with other metabolic pathways or by production of toxic compounds. Our previous results showed that mice overexpressing SSAT (SSAT mice) developed a myeloproliferative disease and the bone marrow microenvironment partly contributed to its development. In this study, the physiological role of SSAT and polyamines in bone remodeling was characterized. Skeletal development of the SSAT mice appeared outwardly similar to wild-type mice until maturity, after which the SSAT mice developed kyphosis. With aging, the SSAT overexpression elicited increased bone perimeter with strikingly thinned cortical bone, decreased trabecular thickness and increased trabecular number in mice. In vitro studies showed that the maturation of SSAT overexpressing osteoblasts was impaired and the expression of bone formation marker genes was dramatically decreased. The polyamine pattern in osteoblasts of SSAT mice was distorted in comparison with wild-type mice. However, treatment of osteoblasts with a SSAT-inducing functional polyamine analogue suggested that defective osteoblastogenesis resulted rather from other consequences of enhanced SSAT activity than lowered levels of the higher polyamines. In comparison to SSAT overexpressing mice, SSAT deficiency led to opposite changes in osteoblastogenesis and differences in bone phenotype in mice. In conclusion, the level of SSAT enzyme activity affected osteoblastogenesis and hence influenced bone remodeling and the bone phenotype in mice. Furthermore, our results suggest the contribution of the catabolic part of the polyamine cycle, other than polyamine depletion, in pathophysiological processes of bone remodeling.






Similar content being viewed by others
Abbreviations
- αMetSPD:
-
α-Methylspermidine
- ALP:
-
Alkaline phosphatase
- APAO:
-
Acetylpolyamine oxidase
- BMD:
-
Bone mineral density
- B.Pm:
-
Bone perimeter
- BSP:
-
Bone sialoprotein
- BV:
-
Bone volume
- Cs.Th:
-
Cortical thickness
- Ct.Ar:
-
Cortical area
- CTSK:
-
Cathepsin K
- CTX:
-
Carboxy-terminal cross-linking telopeptide
- MSC:
-
Mesenchymal stromal cell
- NFATC1:
-
Nuclear factor of activated T-cells cytoplasmic 1
- OC:
-
Osteocalcin
- ODC:
-
Ornithine decarboxylase
- OPN:
-
Osteopontin
- OSX:
-
Osterix
- PINP:
-
Procollagen type I propeptide
- Po.V:
-
Total volume of porosity
- ROS:
-
Reactive oxygen species
- RUNX2:
-
Runt-related transcription factor 2
- SMI:
-
Structural model index
- SSAT:
-
Spermidine/spermine N 1-acetyltransferase
- SSATKO:
-
Spermidine/spermine N 1-acetyltransferase knockout
- Tb.Th:
-
Trabecular thickness
- Tb.N:
-
Trabecular number
- Tb.Sp:
-
Trabecular separation
- TRAcP:
-
Tartrate-resistant acid phosphatase
- Tt.Ar:
-
Total periosteal area
- TV:
-
Tissue volume
References
Adolfsson J, Borge OJ, Bryder D et al (2001) Upregulation of Flt3 expression within the bone marrow lin(-)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity 15(4):659–669
Adolfsson J, Mansson R, Buza-Vidas N et al (2005) Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell 121(2):295–306
Chen C, Young BA, Coleman CS, Pegg AE, Sheppard D (2004) Spermidine/spermine N1-acetyltransferase specifically binds to the integrin alpha9 subunit cytoplasmic domain and enhances cell migration. J Cell Biol 167(1):161–170
Choo MK, Yeo H, Zayzafoon M (2009) NFATc1 mediates HDAC-dependent transcriptional repression of osteocalcin expression during osteoblast differentiation. Bone 45(3):579–589
Clarke B (2008) Normal bone anatomy and physiology. Clin J Am Soc Nephrol 3(Suppl 3):S131–S139
deHart GW, Jin T, McCloskey DE, Pegg AE, Sheppard D (2008) The alpha9beta1 integrin enhances cell migration by polyamine-mediated modulation of an inward-rectifier potassium channel. Proc Natl Acad Sci USA 105(20):7188–7193
DeKoter RP, Singh H (2000) Regulation of B lymphocyte and macrophage development by graded expression of PU.1. Science 288(5470):1439–1441
Edwards CM, Zhuang J, Mundy GR (2008) The pathogenesis of the bone disease of multiple myeloma. Bone 42(6):1007–1013
Eisenberg T, Knauer H, Schauer A et al (2009) Induction of autophagy by spermidine promotes longevity. Nat Cell Biol 11(11):1305–1314
Eliades A, Matsuura S, Ravid K (2012) Oxidases and reactive oxygen species during hematopoiesis: a focus on megakaryocytes. J Cell Physiol 227(10):3355–3362
Eriksen EF (2010) Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord 11(4):219–227
Fogg DK, Sibon C, Miled C et al (2006) A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 311(5757):83–87
Fredericq E, Hacha R, Colson P, Houssier C (1991) Condensation and precipitation of chromatin by multivalent cations. J Biomol Struct Dyn 8(4):847–865
Garnero P (2008) Biomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction and therapy monitoring. Mol Diagn Ther 12(3):157–170
Graf T, Enver T (2009) Forcing cells to change lineages. Nature 462(7273):587–594
Grassinger J, Haylock DN, Storan MJ et al (2009) Thrombin-cleaved osteopontin regulates hemopoietic stem and progenitor cell functions through interactions with alpha9beta1 and alpha4beta1 integrins. Blood 114(1):49–59
Janne J, Alhonen L, Pietila M et al (2006) Genetic manipulation of polyamine catabolism in rodents. J Biochem 139(2):155–160
Lai AY, Kondo M (2006) Asymmetrical lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. J Exp Med 203(8):1867–1873
Laiosa CV, Stadtfeld M, Graf T (2006) Determinants of lymphoid-myeloid lineage diversification. Annu Rev Immunol 24:705–738
Lee SB, Park JH, Folk JE et al (2011) Inactivation of eukaryotic initiation factor 5A (eIF5A) by specific acetylation of its hypusine residue by spermidine/spermine acetyltransferase 1 (SSAT1). Biochem J 433(1):205–213
Leeming DJ, Alexandersen P, Karsdal MA, Qvist P, Schaller S, Tanko LB (2006) An update on biomarkers of bone turnover and their utility in biomedical research and clinical practice. Eur J Clin Pharmacol 62(10):781–792
Liu W, Toyosawa S, Furuichi T et al (2001) Overexpression of Cbfa1 in osteoblasts inhibits osteoblast maturation and causes osteopenia with multiple fractures. J Cell Biol 155(1):157–166
Long F (2011) Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol 13(1):27–38
Maatta JA, Buki KG, Gu G et al (2013) Inactivation of estrogen receptor alpha in bone-forming cells induces bone loss in female mice. FASEB J 27(2):478–488
Pirinen E, Kuulasmaa T, Pietila M et al (2007) Enhanced polyamine catabolism alters homeostatic control of white adipose tissue mass, energy expenditure, and glucose metabolism. Mol Cell Biol 27(13):4953–4967
Pirnes-Karhu S, Mantymaa P, Sironen R et al (2013) Enhanced polyamine catabolism disturbs hematopoietic lineage commitment and leads to a myeloproliferative disease in mice overexpressing spermidine/spermine N-acetyltransferase. Amino Acids 46(3):689–700
Pirnes-Karhu S, Mantymaa P, Sironen R et al (2014) Enhanced polyamine catabolism disturbs hematopoietic lineage commitment and leads to a myeloproliferative disease in mice overexpressing spermidine/spermine N(1)-acetyltransferase. Amino Acids 46(3):689–700
Pittenger MF, Mackay AM, Beck SC et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Tabor H (1962) The protective effect of spermine and other polyamines against heat denaturation of deoxyribonucleic acid. Biochemistry 1:496–501
Teven CM, Liu X, Hu N et al (2011) Epigenetic regulation of mesenchymal stem cells: a focus on osteogenic and adipogenic differentiation. Stem Cells Int 2011:201371
Tripathi AK, Chaturvedi R, Ahmad R et al (2002) Peripheral blood leucocytes ornithine decarboxylase activity in chronic myeloid leukemia patients: prognostic and therapeutic implications. Leuk Res 26(4):349–354
Veeravalli KK, Ponnala S, Chetty C, Tsung AJ, Gujrati M, Rao JS (2012) Integrin alpha9beta1-mediated cell migration in glioblastoma via SSAT and Kir4.2 potassium channel pathway. Cell Signal 24(1):272–281
Venezia TA, Merchant AA, Ramos CA et al (2004) Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLoS Biol 2(10):e301
Vicente C, Conchillo A, Garcia-Sanchez MA, Odero MD (2012) The role of the GATA2 transcription factor in normal and malignant hematopoiesis. Crit Rev Oncol Hematol 82(1):1–17
Vincent A, Crozatier M (2010) Neither too much nor too little: reactive oxygen species levels regulate drosophila hematopoiesis. J Mol Cell Biol 2(2):74–75
Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL (2004) Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 103(9):3258–3264
Zhang J, Niu C, Ye L et al (2003) Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425(6960):836–841
Zhang W, Ou G, Hamrick M et al (2008) Age-related changes in the osteogenic differentiation potential of mouse bone marrow stromal cells. J Bone Miner Res 23(7):1118–1128
Zhao Q, Wang X, Liu Y, He A, Jia R (2010) NFATc1: functions in osteoclasts. Int J Biochem Cell Biol 42(5):576–579
Zhu S, Ashok M, Li J et al (2009) Spermine protects mice against lethal sepsis partly by attenuating surrogate inflammatory markers. Mol Med 15(7–8):275–282
Acknowledgments
We gratefully acknowledge Ms Tuula Reponen, Ms Anne Karppinen, Ms Arja Korhonen and Ms Marita Heikkinen for their skilful technical assistance. We thank Professor Alex Khomutov for providing alpha-methylated spermidine analogue.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pirnes-Karhu, S., Määttä, J., Finnilä, M. et al. Overexpression of spermidine/spermine N 1-acetyltransferase impairs osteoblastogenesis and alters mouse bone phenotype. Transgenic Res 24, 253–265 (2015). https://doi.org/10.1007/s11248-014-9836-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-014-9836-6