Abstract
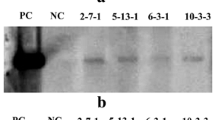
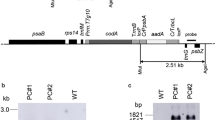
Maize is a typical C4 plant of the NADP-malic enzyme type, and its high productivity is supported by the C4 photosynthetic cycle, which concentrates atmospheric CO2 in the leaves. The plant exhibits superior photosynthetic ability under high light and high temperature, but under cold conditions the photosynthetic rate is significantly reduced. Pyruvate orthophosphate dikinase (PPDK), a key enzyme of the C4 pathway in maize, loses its activity below about 12 °C by dissociation of the tetramer and it is considered as one possible cause of the reduction in the photosynthetic rate of maize at low temperatures. To improve the cold stability of the enzyme, we introduced a cold-tolerant PPDK cDNA isolated from Flaveria brownii into maize by Agrobacterium-mediated transformation. We obtained higher levels of expression by using a double intron cassette and a chimeric cDNA made from F. bidentis and F. brownii with a maximum content of 1 mg/g fresh weight. In leaves of transgenic maize, PPDK molecules produced from the transgene were detected in cold-tolerant homotetramers or in heterotetramers of intermediate cold susceptibility formed with the internal PPDK. Simultaneous introduction of an antisense gene for maize PPDK generated plants in which the ratio of heterolologous and endogenous PPDK was greatly improved. Arrhenius plot analysis of the enzyme extracted from one such plant revealed that the break point was shifted about 3 °C lower than that of the wild type.
Similar content being viewed by others
References
Ashton AR, Burnell JN and Hatch MD (1984) Regulation of C4 photosynthesis: inactivation of pyruvate, Pi dikinase by ADP-dependent phosphorylation and activation by phosphorolysis. Arch Biochem Biophys 230: 492-503.
Ashton AR, Furbank RT and Trevanion SJ (1999) Antisense RNA inhibition of pyruvate, orthophosphate dikinase and NADP malate dehydrogenase in the C4 plant Flaveria bidentis: analysis of plants with a mosaic phenotype. Aust J Plant Physiol 26: 537-547.
Bensen RJ, Johal GS, Crane VC, Tossberg JT, Schnable PS et al. (1995) Cloning and characterization of the maize an1 gene. Plant Cell 7: 75-84.
Burnell JN (1990) A comparative study of the cold-sensitivity of pyruvate, Pi dikinase in Flaveria species. Plant Cell Physiol 31: 295-297.
Burnell JN and Hatch MD (1985) Regulation of C4 photosynthesis: purification and properties of the protein catalyzing ADP-mediated inactivation and Pi-mediated activation of pyruvate, Pi dikinase. Arch Biochem Biophys 237: 490-503.
Denecke J, Gosele J, Botterman J and Cornelissen M (1989) Quantitative analysis of transiently expressed gene in plant cells. Method Mol Cell Biol 1: 19-27.
Du Y-C, Nose A and Wasano K (1999a) Effects of chilling temperature on photosynthetic rates, photosynthetic enzyme activities and metabolite levels in leaves of three sugarcane species. Plant Cell Environ 22: 317-324.
Du Y-C, Nose A and Wasano K (1999b) Thermal characteristics of C4 photosynthetic enzymes from leaves of three sugarcane species differing in cold sensitivity. Plant Cell Physiol 40: 298-304.
Fukayama H, Tsuchida H, Agarie S, Nomura M, Onodera H et al. (2001) Significant accumulation of C4-specific pyruvate, orthophosphate dikinase in a C3 plant, rice. Plant Physiol 127: 1136-1146.
Furbank RT, Chitty JA, Jenkins CLD, Taylor WC, Trevanion SJ et al. (1997) Genetic manipulation of key photosynthetic enzymes in the C4 plant Flaveria bidentis. Aust J Plant Physiol 24: 477-485.
Getzoff TP, Zhu G, Bohnert HJ and Jensen RG (1998) Chimeric Arabidopsis thaliana ribulose-1,5-bisphosphate carboxylase/oxygenase containing a pea small subunit protein is compromised in carbamylation. Plant Physiol 116: 695-702.
Glackin CA and Grula JW (1990) Organ-specific transcripts of different size and abundance derive from the same pyruvate, orthophosphate dikinase gene in maize. Proc Natl Acad Sci USA 87: 3004-3008.
Hatch MD (1979) Regulation of C4 carbon pathway photosynthesis: factors affecting cold mediated inactivation and reactivation of pyruvate orthophosphate dikinase EC-2.7.9.1. Aust J Plant Physiol 6: 607-619.
Hatch MD (1987) C4 photosynthesis: a unique blend of modi-fied biochemistry, anatomy and ultrastructure. Biochim Biophys Acta 895: 81-106.
Hudspeth RL and Grula JW (1989) Structure and expression of the maize gene encoding the phosphoenolpyruvte carboxylase isozyme involved in C4 photosynthesis. Plant Mol Biol 12: 579-589.
Ishida Y, Saito H, Ohta S, Hiei Y, Komari T et al. (1996) High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nat Biotechnol 14: 745-750.
Jenkins CL and Hatch MD (1985) Properties and reaction mechanism of C4 leaf pyruvate, Pi dikinase. Arch Biochem Biophys 239: 53-62.
Komari T, Hiei Y, Saito Y, Murai N and Kumashiro T (1996) Vectors carrying two separate T-DNAs for co-transformation of higher plants mediated by Agrobacterium tumefaciens and segregation of transformants free from selection markers. Plant J 10: 165-174.
Ku MS, Agarie S, Nomura M, Fukayama H, Tsuchida H et al. (1999) High-level expression of maize phosphoenolpyruvate carboxylase in transgenic rice plants. Nat Biotechnol 17: 76-80.
Maruta Y, Ueki J, Saito H, Nitta N and Imaseki H (2002) Transgenic rice with reduced glutelin content by transformation with glutelin A antisense gene. Mol Breeding 8: 273-284.
Matsuoka M (1990) Structure, genetic mapping, and expression of the gene for pyruvate, orthophosphate dikinase from maize. J Biol Chem 265: 16772-16777.
Matsuoka M and Numazawa T (1991) Cis-acting elements in the pyruvate, orthophosphate dikinase gene from maize. Mol Gen Genet 228: 143-152.
Ohta S, Mita S, Hattori T and Nakamura K (1990) Construction and expression in tobacco of a b-glucuronidase (GUS) reporter gene containing an intron within the coding sequence. Plant Cell Physiol 31: 805-813.
Ohta S, Usami S, Ueki J, Kumashiro T, Komari T et al. (1996) Identification of the amino acid residues responsible for cold tolerance in Flaveria brownii pyruvate, orthophosphate dikinase. FEBS Lett 396: 152-156.
Sheen J (1991) Molecular mechanisms underlying the differential expression of maize pyruvate, orthophosphate dikinase genes. Plant Cell 3: 225-245.
Shirahashi K, Hayakawa S and Sugiyama T (1978) Cold lability of pyruvate, orthophosphate dikinase in the maize leaf. Plant Physiol 62: 826-830.
Simon J-P (1996) Molecular forms and kinetic properties of pyruvate, Pi dikinase from two populations of barnyard grass (Echinochloa crus-galli) from sites of contrasting climates. Aust J Plant Physiol 23: 191-199.
Sugiyama T (1973) Purification, molecular, and catalytic properties of pyruvate phosphate dikinase from the maize leaf. Biochemistry 12: 2862-2868.
Sugiyama T and Boku K (1976) Differing sensitivity of pyruvate orthophosphate dikinase to low temperature in maize cultivars. Plant Cell Physiol 17: 851-854.
Sugiyama T and Hirayama Y (1983) Correlation of activities of phosphoenolpyruvate carboxylase and pyruvate, orthophosphate dikinase with biomass in maize seedlings. Plant Cell Physiol 24: 783-787.
Taniguchi M, Izawa K, Ku MS, Lin JH, Saito H et al. (2000a) The promoter for the maize C4 pyruvate, orthophosphate dikinase gene directs cell-and tissue-specific transcription in transgenic maize plants. Plant Cell Physiol 41: 42-48.
Taniguchi M, Izawa K, Ku MS, Lin JH, Saito H et al. (2000b) Binding of cell type-specific nuclear proteins to the 50-flanking region of maize C4 phosphoenolpyruvate carboxylase gene confers its differential transcription in mesophyll cells. Plant Mol Biol 44: 543-557.
Terada R, Urawa H, Inagaki Y, Tsugane K and Iida S (2002) Efficient gene targeting by homologous recombination in rice. Nat Biotechnol 20: 1030-1034.
Ueda K and Sugiyama T (1976) Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol 57: 906-910.
Ueki J, Ohta S, Morioka S, Komari T, Kuwata S et al. (1999) The synergistic effects of two-intron insertions on heterologous gene expression and advantages of the first intron of a rice gene for phospholipase D. Plant Cell Physiol 40: 618-623.
Usami S, Ohta S, Komari T and Burnell JN (1995) Cold stability of pyruvate, orthophosphate dikinase of Flaveria brownii. Plant Mol Biol 27: 969-980.
Usuda H (1984) Variations in the photosynthesis rate and activity of photosynthetic enzymes in maize leaf tissue of different ages. Plant Cell Physiol 25: 1297-1301.
Usuda H, Ku MSB and Edwards GE (1984a) Activation of NADP-malate dehydrogenase, pyruvate, Pi dikinase, and fructose 1,6-bisphosphatase in relation to photosynthetic rate in maize. Plant Physiol 76: 238-243.
Usuda H, Ku MSB and Edwards GE (1984b) Rates of photosynthesis relative to activity of photosynthetic enzymes chlorophyll and soluble protein content among ten 4-carbon pathway species. Aust J Plant Physiol 11: 509-517.
Ward DA (1987) The temperature acclimation of photosynthetic response to CO2 in Zea mays and its relationship to the activities of photosynthetic enzymes and the CO2-concentrating mechanism of C4 photosynthesis. Plant Cell Environ 10: 407-411.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ohta, S., Ishida, Y. & Usami, S. Expression of cold-tolerant pyruvate, orthophosphate dikinase cDNA, and heterotetramer formation in transgenic maize plants. Transgenic Res 13, 475–485 (2004). https://doi.org/10.1007/s11248-004-1452-4
Issue Date:
DOI: https://doi.org/10.1007/s11248-004-1452-4