Abstract
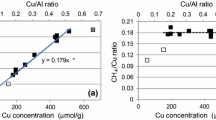
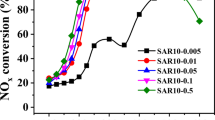
The effect of NH4OH/Cu2+ in a copper-acetate solution on the properties of ion-exchanged Cu-ZSM-5 catalysts in the selective catalytic reduction of NO by NH3 (NO–NH3 SCR) has been studied. The temperature programmed desorption of ammonia (NH3-TPR) on Cu-ZSM-5 and the ammonia adsorption–desorption dynamics at 75–300 °C were studied to identify and quantify the nature of acid sites and ammonia desorption heat of Cu-ZSM-5. The Cu-ZSM-5 catalysts containing Cu-structures with extra-lattice oxygen were active in the low-temperature SCR, whereas those with isolated Cu2+ ions were active in the high-temperature SCR. It was shown that Cu-structures with extra-lattice oxygen were generated during the ion exchange of H-ZSM-5 with a water-ammonia solution of copper-acetate where the NH4OH/Cu2+ ratio was in the range of 6–15. Isolated Cu2+ ions were produced in the ion-exchange mode with the ammonia-free solution.







Similar content being viewed by others
References
Li J, Chang H, Ma L, Hao J, Yang RT (2011) Catal Today 175:147–156
Komatsu T, Nunokawa M, Moon S, Takahara T, Namba S, Yashima T (1994) J Catal 148:427–437
Sullivan JA, Cunningham J, Morris MA, Keneavey K (1995) Appl Catal B 7:137–151
Salker AV, Weisweiler W (2000) Appl Catal A 203:221–229
Choi EY, Nam IS, Kim YG (1996) J Catal 161:597–604
Ramachandran B, Herman RG, Choi S, Stenger HG, Lyman CE, Sale JW (2000) Catal Today 55:281–290
Baik JH, Yim SD, Nam IS, Mok YS, Lee JH, Cho BK, Oh SH (2004) Top Catal 30–31:37–42
Rahkamaa-Tolonen K, Maunula T, Lomma M, Huuhtanen M, Keiski RL (2005) Catal Today 100:217–222
Sjovall H, Olsson L, Fridell E, Blint RJ (2006) Appl Catal B 64:180–188
Park J-H, Parka HJ, Baik JH, Nam I-S, Shin C-H, Lee J-H, Cho BK, Oh SH (2006) J Catal 240:47–57
Sultana A, Nanba T, Haneda M, Sasaki M, Hamada H (2010) Appl Catal B Environ 101:61–67
Wilken N, Wijayanti K, Kamasamudram K, Currier NW, Vedaiyan R, Yezerets A, Olsson L (2012) Appl Catal B 111–112:58–66
Kwak JH, Tonkyn RG, Kim DH, Szanyi J, Peden CHF (2010) J Catal 275:187–190
Fickel DW, D’Addio E, Lauterbach JA, Lobo RF (2011) Appl Catal B 102:441–448
Deka U, Juhin A, Eilertsen EA, Emerich H, Green MA, Korhonen ST, Weckhuysen BM, Beale AM (2012) J Phys Chem C 116:4809–4818
Wang L, Li W, Qi GS, Weng D (2012) J Catal 289:21–29
Wang J, Yu T, Wang XQ, Qi GS, Xue JJ, Shen MQ, Li W (2012) Appl Catal B 127:137–147
Metkar PS, Harold MP, Balakotaiah V (2013) Chem Eng Sci 87:51–66
Goltl F, Bulo RE, Hafner J, Sautet P (2013) J Phys Chem Lett 2244–2249
Bates SA, Verma AA, Paolucci C, Parekh AA, Anggara T, Yezerets A, Schneider WF, Miller JT, Delgass WN, Ribeiro FH (2014) J Catal 312:87–97
Joshi SY, Kumar A, Luo J, Kamasamudram K, Yezerets A (2015) Appl Catal B 165:27–35
Wang D, Jangjou Y, Liu Y, Sharma MK, Luo J, Li J, Kamasamudram K, Epling WS (2015) Appl Catal B 165:438–445
Gao F, Walter ED, Kollar M, Wang Y, Szanyi J, Peden CHF (2014) J Catal 319:1–14
Kieger S, Delahay G, Coq B, Neveu B (1999) J Catal 183:267–280
Sultana A, Sasaki M, Suzuki K, Hamada H (2013) Catal Commun 41:21–25
Yashnik SA, Ismagilov ZR, Anufrienko VF (2005) Catal Today 110:310–322
Yashnik SA, Salnikov AV, Vasenin NT, Anufrienko VF, Ismagilov ZR (2012) Catal Today 197:214–227
Yashnik SA, Ismagilov ZR (2015) Appl Catal B 170–171:241–254
Yashnik SA, Ismagilov ZR (2016) Kin Catal 57:776–796
Cvetanovic RJ, Amenomiya Y (1972) Catal Rev 6:21–48
Baes CF Jr, Mesmer RE (1976) The Hydrolysis of cations. Wiley, New York, 267–274
Zhang T, Shi J, Liu J, Wang D, Zhao Z, Cheng K, Li (2016) Appl Surf Sci 375:186–195
Katada N, Igi H, Kim J, Niwa M (1997) J Phys Chem B 110:5969–5977
Martins GVA, Berlier G, Bisio C, Coluccia S, Pastore HO, Marchese L (2008) J Phys Chem C 112:7193–7200
Huang Y, Vansant EF (1973) J Phys Chem 77:663–667
Vansant EF, Lunsford JH (1972) J Phys Chem 76:2860–2865
Luo J, Gao F, Kamasamudram K, Currier N, Peden CHF, Yezerets A (2017) J Catal 348:291–299
Colombo M, Nova I, Tronconi E (2010) Catal Today 151:223–230
Ruggeri MP, Nova I, Tronconi E, Collier JE, York APE (2016) Top Catal 59:875–881
Lever ABP (1984) Inorganic Electron Spectroscopy. 2, Elsevier, Amsterdam
Tikhomirova NN, Zamaraev KI, Berdnikov VM (1963) J Struct Chem (Engl Transl) 4:407–411
Bendrich M, Scheuer A, Hayes RE, Votsmeier M (2018) Appl Catal B 222:76–87
Zhu H, Kwak JH, Peden CHF, Szanyi J (2013) Catal Today 205:16–23
Koebel M, Elsener M (1998) Chem Eng Sci 53:657–669
Bates SA, Delgass WN, Ribeiro FH, Miller JT, Gouder R (2014) J Catal 312:26–36
Pereda-Ayo B, De La Torre U, Illan-Gomez MJ, Bueno-Lopez A, Gonzalez-Velasco JR (2014) Appl Catal B 147:420–428
Acknowledgements
The work was conducted within the framework of the budget project (АААА-А17-117041710086-6) of Boreskov Institute of Catalysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yashnik, S.A., Ismagilov, Z.R. Control of the NO–NH3 SCR Behavior of Cu-ZSM-5 by Variation of the Electronic State of Copper. Top Catal 62, 179–191 (2019). https://doi.org/10.1007/s11244-018-1101-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-018-1101-4