Abstract
Plasmas and gas discharges in contact with liquids have played an important role in the history of chemical processing and scientific inquiry, leading to the discoveries of elements such as argon and compounds such as ozone. Recently-developed atmospheric-pressure plasma sources have renewed the study of plasma–liquid systems with applications in chemical processing, materials synthesis, and chemical analysis. In many cases, these approaches utilize glow discharge electrolysis configurations where a DC plasma replaces one of the metal electrodes in a standard electrolytic cell. These configurations have been used to great effect for the synthesis of various nanomaterials and more recently, in the processing of carbon dioxide. In this work, we overview recent developments using plasmas as electrodes in electrolytic cells for chemical processing, drawing parallels to conventional electrochemistry and electrocatalysis. In particular, we highlight recent studies on the fundamental chemical processes at the plasma–liquid interface, including new interfacial measurement techniques used to probe charge transfer. We conclude with an overview of opportunities for these configurations in the future and highlight the need for further fundamental study.


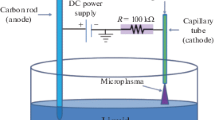
Courtesy of R. Mohan Sankaran, Case Western Reserve University


Adapted with permission from [51]. Copyright (2011) American Chemical Society

Copyright 2014 IEEE. Adapted, with permission, from [54]

Reprinted with permission from [55]. Copyright 2013 American Chemical Society

Used with permission from [56]

Used with permission from [60]
Similar content being viewed by others
References
Qiao J, Liu Y, Hong F, Zhang J (2014) A review of catalysts for the electro reduction of carbon dioxide to produce low-carbon fuels. Chem Soc Rev 43:631
Ni M, MKH Leung, DYC Leung, Sumathy K (2007) A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew Sustain Energy Rev 11:401
Liu H, Song C, Zhang L, Zhang J, Wang H, Wilkinson DP (2006) A review of anode catalysis in the direct methanol fuel cell. J Power Sources 155:95
SKS Gupta (2015) Contact glow discharge electrolysis: its origin, plasma diagnostics and non-faradaic chemical effects. Plasma Sources Sci Technol 24:063001
Cavendish H (1784) Experiments on air. Phil Trans Royal Soc London 75:372
Rayleigh L, Ramsay W (1895) Argon, a new constituent of the atmosphere. Proc Roy Soc London 57:265
Schönbein CF (1840) Beobachtungen über den bei der Elektrolysation des Wassers und dem Ausströmen der gewöhnliehen Elektricität aus Spitzen sich entwikkelnden Geruch. Ann Phys 126:616
Birkeland KR (1889) On the oxidation of atmospheric nitrogen in electric arcs. Nature 58:98
Gubkin J (1887) Electrolytische Metallabscheidung an der freien Oberfläche einer Salzlösung. Ann Phys Chem 32:114
Klemenc A (1927) Zur Kenntnis der elektrolytischen Reduktion und der Reaktionen im Glimmbogen an der Phasengrenze Flüssigkeit-Gas. Z Physik Chem A 130:378
Bruggeman P, Leys C (2009) Non-thermal plasmas in and in contact with liquids. J Phys D 42:053001
Mariotti D, Sankaran RM (2010) Microplasmas for nanomaterials synthesis. J Phys D 43:323001
Hickling A (1971) Electrochemical processes in glow discharge at the gas-solution interface. In: Bockris JO’M, Conway BE (eds) Modern aspects of electrochemistry, vol 6. Springer, New York, pp 329–373
Denaro AR, Hickling A (1958) Glow-discharge electrolysis in aqueous solutions. J Electrochem Soc 105:265
Cserfalvi T, Mezei P, Apai P (1993) Emission studies on a glow discharge in atmospheric pressure air using water as a cathode. J Phys D 26:2184
Mezei P, Cserfalvi T (2007) Electrolyte cathode atmospheric glow discharges for direct solution analysis. Appl Spectrosc Rev 42:573
Webb MR, Hieftje GM (2009) Spectrochemical analysis by using discharge devices with solution electrodes. Anal Chem 81:862
Jamorz P, Greda K, Pohl P (2012) Development of direct-current, atmospheric-pressure, glow discharges generated in contact with flowing electrolyte solutions for elemental analysis by optical emission spectrometry. Trends Anal Chem 41:105
Schwarz AJ, Ray SJ, Hieftje GM (2015) Automatable on-line generation of calibration curves and standard additions in solution-cathode glow discharge optical emission spectrometry. Spectrochim Acta Part B 105:77–83
Richmonds C, Sankaran RM (2008) Plasma-solution electrochemistry: rapid synthesis of colloidal metal NPs by microplasma reduction of aqueous cations. Appl Phys Lett 93:131501
Chen Q, Kaneko T, Hatakeyama R (2012) Reductants in gold nanoparticle synthesis using gas–liquid interfacial discharge plasmas. Appl Phys Express 5:086201
Patel J, Nemcova L, Maguire P, Graham WG, Mariotti D (2013) Synthesis of surfactant-free electrostatically stabilized gold nanoparticles by plasma-inducted liquid chemistry. Nanotechnology 24:245604
Shirai N, Uchida S, Tochikubo F (2014) Synthesis of metal nanoparticles by dual plasma electrolysis using atmospheric dc glow discharge in contact with liquid. Jpn J Appl Phys 53:046202
Brettholle M, Hofft O, Klarhofer L, Mathes S, Maus-Friedrichs W, Zein El Abedin S, Krischok S, Janek J, Endres F (2010) Plasma electrochemistry in ionic liquids: deposition of copper nanoparticles. Phys Chem Chem Phys 12:1750–1755
Kulbe N, Hofft O, Ulbrich A, Zein El Abedin S, Krischok S, Janek J, Polleth M, Endres F (2011) Plasma electrochemistry in 1-butyl-3-methylimidazolium dicyanamide: copper nanoparticle from CuCl and CuCl2. Plasma Processes Polym 8:32–37
Hofft O, Endres F (2011) Plasma electrochemistry in ionic liquids: an alternative route to generate nanoparticles. Phys Chem Chem Phys 13:13472–13478
Kaneko T, Baba K, Hatakeyama R (2009) Gas–liquid interfacial plasmas: basic properties and applications to nanomaterial synthesis. Plasma Phys Control Fusion 51:124011
Lee SW, Janyasupab M, Liu C-C, Sankaran RM (2013) Fabrication of Ir nanoparticle-based biosensors by plasma electrochemical reduction for enzyme-free detection of hydrogen peroxide. Catal Today 211:137–142
Endres F, Hofft O, von Brisinski NS (2014) Plasma electrochemistry in ionic liquids: from silver to silicon nanoparticles. J Mol Liq 192:59–66
Ghosh S, Bishop B, Morrison I, Akolkar R, Scherson D, Sankaran RM (2015) Generation of a direct-current, atmospheric-pressure microplasma at the surface of a liquid water microjet for continuous plasma–liquid processing. J Vaccum Sci Technol A 33:021312
Mariotti D, Švrček V, Hamilton JW, Schmidt M, Kondo M (2012) Silicon nanocrystals in liquid media: optical properties and surface stabilization by microplasma-induced non-equilibrium liquid chemistry. Adv Funct Mater 22:954–964
McKenna J, Patel J, Mitra S, Soin N, Švrček V, Maguire P, Mariotti D (2011) Synthesis and surface engineering of nanomaterials by atmospheric-pressure microplasmas. Euro Phys J 56:24020
Chen Q, Li J, Li Y (2015) A review of plasma-liquid interactions for nanomaterial synthesis. J Phys D 48:424005
Mezei P, Cserfalvi T (2012) A critical review of published data on the gas temperature and the electron density in the electrolyte cathode atmospheric glow discharges. Sensors 12:6576–6586
Oehmigen K, Hähnel M, Brandenburg R, Wilke C, Weltmann K-D, von Woedtke T (2010) The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Process Polym 7:250
CAJ van Gils, Hofmann S, BKHL Boekema, Brandenburg R, Bruggeman PJ (2013) Mechanisms of bacterial inactivation in the liquid phase induced by a remote RF cold atmospheric pressure plasma jet. J Phys D 46:175203
Locke BR, Shih K-Y (2011) Review of the methods to form hydrogen peroxide in electrical discharge plasma with liquid water. Plasma Sources Sci Technol 20:034006
Vasko CA, Liu DX, vanVeldhuizen EM, Iza F, Bruggeman PJ (2014) Hydrogen peroxide production in an atmospheric pressure RF glow discharge: comparison of models and experiments. Plasma Chem Plasma Process 34:1081–1099
Graves DB (2012) The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J Phys D 45:263001
Norberg SA, Tian W, Johnsen E, Kushner MJ (2014) Atmospheric pressure plasma jets interacting with liquid covered tissue: touching and not-touching the liquid. J Phys D 47:475203
Liu ZC, Liu DX, Chen C, Li D, Yang AJ, Rong MZ, Chen HL, Kong MG (2015) Physicochemical processes in the indirect interaction between surface air plasma and deionized water. J Phys D 48:495201
Liu DX, Liu ZC, Chen C, Yang AJ, Li D, Rong MZ, Chen HL, Shama G, Kong MG (2016) Aqueous reactive species induced by a surface air discharge: heterogeneous mass transfer and liquid chemistry pathways. Sci Rep 6:23737
Lindsey AD, Graves DB, Shannon SC (2016) Fully coupled simulation of the plasma liquid interface and interfacial coefficient effects. J Phys D 49:235204
Yusupov M, Neyts EC, Simon P, Berdiyorov G, Snoeckx R, ACT van Duin, Bogaerts A (2014) Reactive molecular dynamics simulations of oxygen species in a liquid water layer of interest for plasma medicine. J Phys D 47:025205
T Cserfalvi, P Mezei (1996) Operating mechanism of the electrolyte cathode atmospheric glow discharge. Fresen J Anal Chem 355:813–819
Denaro AR, Hough KO (1972) Glow-discharge electrolysis of sulphuric acid solutions. Electrochim Acta 17:549
Khlyustova AV, Maksimov AL, Khorev MS (2008) Radiation of metal atoms in the plasma of an atmospheric pressure glow discharge with an electrolyte cathode. Surf Eng Appl Electrochem 44:370
Marcus RK, Davis WC (2001) An atmospheric pressure glow discharge optical emission source for the direct sampling of liquid media. Anal Chem 73:2903–2910
Schwartz AJ, Ray SJ, Elish E, Storey AP, Rubinshtein AA, Chan GC, Pfeuffer KP, Hieftje GM (2012) Visual observations of an atmospheric-pressure solution-cathode glow discharge. Talanta 102:26
Quarles CD, Gonzalez J, Choi I, Ruiz J, Mao X, Marcus RK, Russo RE (2012) Liquid sampling-atmospheric pressure glow discharge optical emission spectroscopy detection of laser ablation produced particles: a feasibility study. Spectrochim Acta Part B 76:190
Richmonds C, Witzke M, Bartling B, Lee SW, Wainright J, Liu C-C, Sankaran RM (2011) Electron-transfer reactions at the plasma–liquid interface. J Am Chem Soc 133:17582
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O–) in aqueous solution. J Phys Chem Ref Data 17:513–886
Witzke M, Rumbach P, Go DB, Sankaran RM (2012) Evidence for the electrolysis of water by plasmas formed at the surface of aqueous solutions. J Phys D 45:44201
Rumbach P, Griggs N, Sankaran RM, Go DB (2014) Visualization of electrolytic reactions at a plasma–liquid interface. IEEE Trans Plasma Sci 42:2610
Rumbach P, Witzke M, Sankaran RM, Go DB (2013) Decoupling interfacial reactions between plasmas and liquids: charge transfer vs. plasma neutral reactions. J Am Chem Soc 135:16264
Rumbach P, Bartels DM, Sankaran RM, Go DB (2015) The solvation of electrons by an atmospheric pressure plasma. Nat Commun 6:7248
Bartels DM, Takahashi K, Cline JA, Marin TW, Jonah CD (2005) Pulse radiolysis of supercritical water. 3. Spectrum and thermodynamics of the hydrated electron. J Phys Chem A 109:1299
Rumbach P, Bartels DM, Sankaran RM, Go DB, Corrigendum (2015) The solvation of electrons by an atmospheric-pressure plasma. Nat Commun 7:11911
Rumbach P, Bartels DM, Sankaran RM, Go DB (2015) The effect of air on solvated electron chemistry at a plasma/liquid interface. J Phys D 48:424100
Rumbach P, Xu R, Go DB (2016) Electrochemical production of oxalate and formate from CO2 by solvated electrons produced using an atmospheric-pressure plasma. J Electrochem Soc 163:F1157–F1161
Lu Q, Rosen J, Zhou Y, Hutchings GS, Kimmel YC, Chen JG, Jiao F (2014) A selective and efficient electrocatalyst for carbon dioxide reduction. Nat Commun 5
Angamuthu R, Byers P, Lutz M, Spek AL, Bouwman E (2010) Electrocatalytic CO2 conversion to oxalate by a copper complex. Science 327:313–315
Nakata K, Ozaki T, Terashima C, Fujishima A, Einaga Y (2014) High-yield electrochemical production of formaldehyde from CO2 and seawater. Angew Chem 126:890–893
Gordon S, Hart EJ, Matheson MS, Rabani J, Thomas JK (1963) Reactions of the hydrated electron. Discuss Faraday Soc 36:193–205
Fojtik A, Czapski G, Henglein A (1970) Pulse radiolytic investigation of the carboxyl radical in aqueous solution. J Phys Chem 74:3204–3208
Lin M, Katsumura Y, Muroya Y, He H, Miyazaki T, Hiroishi D (2008) Pulse radiolysis of sodium formate aqueous solution up to 400 °C: absorption spectra, kinetics and yield of carboxyl radical \({\text{CO}}_2^{ \bullet - }\). Rad Phys Chem 77:1208–1212
Flyunt R, Schuchmann MN, von Sonntag C (2001) A common carbanion intermediate in the recombination and proton-catalysed disproportionation of the carboxyl radical anion, \({\text{CO}}_2^{ \bullet - },\) in aqueous solution. Chem Eur J 7:796–799
Bhüler RE, Staehelin J, Hoigné J (1984) Ozone decomposition in water studied by pulse radiolysis. 1. Perhydroxyl (HO2)/hyperoxide (\({\text{O}}_2^ -\)) and \({\text{H}}{{\text{O}}_{\text{3}}}/{\text{O}}_3^ -\) as intermediates. J Phys Chem 88:2560–2564
Acknowledgements
We would like to acknowledge our collaborators R. Mohan Sankaran, David M. Bartels, and Megan Witzke who have contributed to our work this area. R. Mohan Sankaran also provided Fig. 2 in this manuscript. The authors’ work has been supported by the US Army Research Office under Award Number W911NF-14-1-0241 and the Electrochemical Society Toyota Young Investigator Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rumbach, P., Go, D.B. Perspectives on Plasmas in Contact with Liquids for Chemical Processing and Materials Synthesis. Top Catal 60, 799–811 (2017). https://doi.org/10.1007/s11244-017-0745-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-017-0745-9