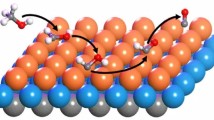
Abstract
Tungsten (5d46s2) and rhenium (5d56s2) form MO3 oxides (M = W or Re) with similar structures. The adsorption and dissociation of methanol on these oxide surfaces, often used to probe the surface redox centers, have been analyzed using periodic density functional calculations. Molecular adsorption of methanol at the metal site on both surfaces with 0.5 ML oxygen coverage was found to be exothermic with adsorption energies of −74 and −106 kJ/mol on WO3(001) and ReO3(001), respectively. In contrast, heterolytic dissociation of methanol to adsorbed methoxide species at the metal site and H at the surface oxygen site is not energetically favored on WO3(001) but favored on ReO3(001). The dissociation energies to form coadsorbed methoxide and hydrogen adatom are 35 kJ/mol on WO3 and −112 kJ/mol on ReO3, respectively. The activation barrier for dissociating the molecularly adsorbed methanol on ReO3(001) was determined to be 19 kJ/mol. Dehydrogenation to form coadsorbed hydroxymethyl and hydrogen adatom is not energetically favorable on both surfaces with respect to the molecularly adsorbed methanol. However, the dehydrogenation path is exothermic on ReO3 with respect to the gas phase methanol, with the heats of reaction of −25 kJ/mol, but highly endothermic on WO3, with the heats of reaction of 114 kJ/mol.









Similar content being viewed by others
References
Henrich VE, Cox PA (1996) The surface science of metal oxides. Cambridge University Press, Cambridge
Murrell LL, Grenoble DC, Kim CJ, Dispenziere NC (1987) Supported transition-metal oxides as acid cracking catalysts—periodic trends and their relationship to activity and selectivity. J Catal 107:463–470
Benitez VM, Querini CA, Figoli NS (2003) Characterization of WOx/Al2O3 and MoOx/Al2O3 catalysts and their activity and deactivation during skeletal isomerization of 1-butene. Appl Catal A 252:427–436
Mamede AS, Payen E, Grange P, Poncelet G, Ion A, Alifanti M, Parvulescu VI (2004) Characterization of WOx/CeO2 catalysts and their reactivity in the isomerization of hexane. J Catal 223:1–12
Di Gregorio F, Keller V (2004) Activation and isomerization of hydrocarbons over WO3/ZrO2 catalysts—I. Preparation, characterization, and X-ray photoelectron spectroscopy studies. J Catal 225:45–55
Benitez VM, Querini CA, Figoli NS, Comelli RA (1999) Skeletal isomerization of 1-butene on WOx/γ-Al2O3. Appl Catal A-Gen 178:205–218
Ji WJ, Chen Y, Kung HH (1997) Vapor phase aldol condensation of acetaldehyde on metal oxide catalysts. Appl Catal A 161:93–104
Hilbrig F, Schmelz H, Knozinger H, Sinev M, Bell AT, Schmal M, Forzatti P, Wachs IE, Uematsu T, Vedrine JC, Hums E (1993) Acidity of WOx/TiO2 catalysts for selective catalytic reduction (SCR). Stud Surf Sci Catal 75:1351–1362
Yuan YH, Iwasawa Y (2002) Performance and characterization of supported rhenium oxide catalysts for selective oxidation of methanol to methylal. J Phys Chem B 106:4441–4449
Viswanadham N, Shido T, Iwasawa Y (2001) Performances of rhenium oxide-encapsulated ZSM-5 catalysts in propene selective oxidation/ammoxidation. Appl Catal A 219:223–233
Barton Cole E, Lakkaraju PS, Rampulla DM, Morris AJ, Abelev E, Bocarsly AB (2010) Using a one-electron shuttle for the multielectron reduction of CO2 to methanol: kinetic, mechanistic, and structural insights. J Am Chem Soc 132:11539–11551
Ge Q (2013) Chapter 3—mechanistic understanding of catalytic CO2 activation from first principles theory. In: Suib SL (ed) New and future developments in catalysis. Elsevier, Amsterdam, pp 49–79
Wachs IE, Deo G, Juskelis MV, Weckhuysen BM (1997) Methanol oxidation over supported vanadium oxide catalysts: new fundamental insights about oxidation reactions over metal oxide catalysts from transient and steady state kinetics. In: Froment GF, Waugh KC (eds) Dynamics of surfaces and reaction kinetics in heterogeneous catalysis. Elsevier, Amsterdam, pp 305–314
Cheng W-H, Kung HH (1994) Methanol production and use. Marcel Dekker, New York
Badlani M, Wachs IE (2001) Methanol: a “smart” chemical probe molecule. Catal Lett 75:137–149
Burcham LJ, Wachs IE (1999) The origin of the support effect in supported metal oxide catalysts: in situ infrared and kinetic studies during methanol oxidation. Catal Today 49:467–484
Wachs IE, Chen Y, Jehng JM, Briand LE, Tanaka T (2003) Molecular structure and reactivity of the group V metal oxides. Catal Today 78:13–24
Gao XT, Jehng JM, Wachs IE (2002) In situ UV-vis-NIR diffuse reflectance and Raman spectroscopic studies of propane oxidation over ZrO2-supported vanadium oxide catalysts. J Catal 209:43–50
Schirber JE, Morosin B (1979) “Compressibility collapse” transition in ReO3. Phys Rev Lett 42:1485–1487
Woodward PM, Sleight AW, Vogt T (1997) Ferroelectric tungsten trioxide. J Solid State Chem 131:9–17
Butler MA, Nasby RD, Quinn RK (1976) Tungsten trioxide as an electrode for photoelectrolysis of water. Solid State Commun 19:1011–1014
Ferretti A, Rogers DB, Goodenough JB (1965) The relation of the electrical conductivity in single crystals of rhenium trioxide to the conductivities of Sr2MgReO6 and NaxWO3. J Phys Chem Solids 26:2007–2011
Gurlo A (2011) Nanosensors: towards morphological control of gas sensing activity. SnO2, In2O3, ZnO and WO3 case studies. Nanoscale 3:154–165
Mei D, Deskins NA, Dupuis M, Ge Q (2008) Density functional theory study of methanol decomposition on the CeO2(110) surface. J Phys Chem C 112:4257–4266
Mei D, Deskins NA, Dupuis M, Ge Q (2007) Methanol adsorption on the clean CeO2(111) surface: a density functional theory study. J Phys Chem C 111:10514–10522
Han Y, Liu C-J, Ge Q (2009) Effect of Pt clusters on methanol adsorption and dissociation over perfect and defective anatase TiO2(101) surface. J Phys Chem C 113:20674–20682
Ye J, Liu C, Ge Q (2012) A DFT study of methanol dehydrogenation on the PdIn(110) surface. Phys Chem Chem Phys 14:16660–16667
Kresse G, Furthmuller J (1996) Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci 6:15–50
Kresse G, Hafner J (1993) Ab initio molecular-dynamics for liquid-metals. Phys Rev B 47:558–561
Vanderbilt D (1990) Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys Rev B 41:7892–7895
Blochl PE (1994) Projector augmented-wave method. Phys Rev B 50:17953–17979
Hobbs D, Kresse G, Hafner J (2000) Fully unconstrained noncollinear magnetism within the projector augmented-wave method. Phys Rev B 62:11556–11570
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Atoms, molecules, solids, and surfaces—applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 46:6671–6687
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13:5188–5192
Mills G, Jonsson H, Schenter GK (1995) Reversible work transition-state theory—application to dissociative adsorption of hydrogen. Surf Sci 324:305–337
Henkelman G, Jonsson H (2000) Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J Chem Phys 113:9978–9985
Henkelman G, Uberuaga BP, Jonsson H (2000) A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J Chem Phys 113:9901–9904
Pan Y-X, Liu C-J, Ge Q (2010) Effect of surface hydroxyls on selective CO2 hydrogenation over Ni4/γ-Al2O3: a density functional theory study. J Catal 272:227–234
Zhang Z, Tang M, Wang Z-T, Ke Z, Xia Y, Park K, Lyubinetsky I, Dohnálek Z, Ge Q (2015) Imaging of formaldehyde adsorption and diffusion on TiO2(110). Top Catal 58:103–113
Kresse G, Furthmuller J (2015) VASP, the Guide
Yakovkin IN, Gutowski M (2007) Driving force for the WO3(001) surface relaxation. Surf Sci 601:1481–1488
Tanner RE, Altman EI (2001) Effect of surface treatment on the γ-WO3(001) surface: a comprehensive study of oxidation and reduction by scanning tunneling microscopy and low-energy electron diffraction. J Vac Sci Technol A 19:1502–1509
Li M, Posadas A, Ahn CH, Altman EI (2005) Scanning tunneling microscopy study of terminal oxygen structures on WO3(100) thin films. Surf Sci 579:175–187
Huang X, Zhai HJ, Li J, Wang LS (2006) On the structure and chemical bonding of tri-tungsten oxide clusters W3O −n and W3On (n = 7 − 10): W3O8 as a potential molecular model for O-deficient defect sites in tungsten oxides. J Phys Chem A 110:85–92
Ling S, Mei D, Gutowski M (2011) Reactivity of hydrogen and methanol on (001) surfaces of WO3, ReO3, WO3/ReO3 and ReO3/WO3. Catal Today 165:41–48
Wachs IE (1996) Raman and IR studies of surface metal oxide species on oxide supports: supported metal oxide catalysts. Catal Today 27:437–455
Macht J, Baertsch CD, May-Lozano M, Soled SL, Wang Y, Iglesia E (2004) Support effects on Bronsted acid site densities and alcohol dehydration turnover rates on tungsten oxide domains. J Catal 227:479–491
Onfroy T, Clet G, Houalla M (2005) Acidity, surface structure, and catalytic performance of WOx supported on monoclinic zirconia. J Phys Chem B 109:3345–3354
Pan Y, Liu C-J, Ge Q (2008) Adsorption and protonation of CO2 on partially hydroxylated γ-Al2O3 surfaces: a density functional theory study. Langmuir 24:12410–12419
Pan Y-X, Kuai P, Liu Y, Ge Q, Liu C-J (2010) Promotion effects of Ga2O3 on CO2 adsorption and conversion over a SiO2-supported Ni catalyst. Energy Environ Sci 3:1322–1325
Pan Y-X, Liu C-J, Mei D, Ge Q (2010) Effects of hydration and oxygen vacancy on CO2 adsorption and activation on β-Ga2O3(100). Langmuir 26:5551–5558
Yin S, Swift T, Ge Q (2011) Adsorption and activation of CO2 over the Cu-Co catalyst supported on partially hydroxylated γ-Al2O3. Catal Today 165:10–18
Acknowledgments
This work was supported by the Office of Basics Energy Sciences, U.S. Department of Energy (DOE) under Grant No. DE-FG02-05ER46231. Calculations was done at the W. R. Wiley Environmental Molecular Sciences Laboratory (EMSL), a national scientific user facility sponsored by DOE’s Office of Biological and Environmental Research and located at PNNL. PNNL is operated by Battelle for the U.S. DOE.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ge, Q., Gutowski, M. A Comparative Study of Methanol Adsorption and Dissociation over WO3(001) and ReO3(001). Top Catal 58, 655–664 (2015). https://doi.org/10.1007/s11244-015-0402-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-015-0402-0