Abstract
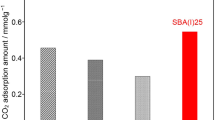
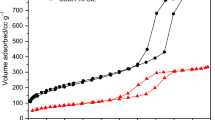
Adsorption of pure CO2 on amine-functionalized SBA-15 mesoporous silica materials has been studied. Adsorbent materials were prepared by grafting the silica surface with aminopropyl (AP), ethylene-diamine (ED) and diethylene-triamine (DT) organosilane molecules. Materials so obtained were dried under air atmosphere at 110 °C and at room temperature. CO2 adsorption isotherms were carried out at 45 °C, showing that grafted materials are very efficient for CO2 removal at atmospheric pressure when samples are dried at 20 º C. However, when the drying step is carried out at 110 °C in air, CO2 adsorption capacity is low. DRIFTS analysis has shown that amino groups can undergo oxidation to oxime or imine species during drying. Adsorption capacity of the materials was found to be unchanged after some consecutive adsorption–desorption cycles, being the regeneration step performed at 110 °C under vacuum.









Similar content being viewed by others
References
IPCC (1990) In: Houghton JT, Jenkins GJ, Ephraims JJ (eds) IPCC first assessment report (FAR). IPCC, New York
Pachauri RK, Reisinger A (eds) (2007) IPCC Fourth Assessment Report: Climate Change 2007 (AR4). IPCC, Geneva
Metz B, Davidson O, de Coninck H, Loos M, Meyer L (eds) (2005) IPCC special report on carbon dioxide capture and storage. IPCC, Cambridge
Kyoto Protocol to the United Nations framework convention on Climate Change. United Nations, 1998
The economics of adaptation to climate change (2009) World Bank, Bangkok
Astarita G (1961) Chem Eng Sci 16:202–207
Maddox RN, Mains GJ, Rahman MA (1987) Ind Eng Chem Res 26:27–31
Rinker EB, Ashour SS, Sandall OC (2000) Ind Eng Chem Res 39:4346–4356
Carbon sequestration. State of Science. (1999) Office of Science and Office of Fossil Energy. US Department of Energy. DOE/OS-FE, Washington DC
Tontiwachwuthikul P, Meisen A, Lim CJJ (1991) Chem Eng Data 36:130–133
Douglas A, Costas T (2005) Sep Sci Technol 40:321–348
Sanz R, Calleja G, Arencibia A, Sanz-Pérez ES (2010) Appl Surf Sci 256:5323–5328
Caplow M (1968) J Am Chem Soc 24:6795–6803
Oye G, Sjoblom J, Stocker M (2001) Adv Colloid Interface Sci 89:439–466
Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH, Chmelka BF, Stucky GD (1998) Science 279:548–552
Xu X, Song C, Andrésen JM, Miller BG, Scaroni AW (2002) Energy Fuel 16:1463–1469
Xu X, Song C, Andrésen JM, Miller BG, Scaroni AW (2003) Microporous Mesoporous Mater 62:29–45
Xu X, Song C, Miller BG, Scaroni AW (2005) Ind Eng Chem Res 44:8113–8119
Wang X, Schwartz V, Clark JC, Ma X, Overbury SH, Xu X, Song C (2009) J Phys Chem C 113:7260–7268
Son WJ, Choi JS, Ahn WS (2008) Microporous Mesoporous Mater 113:31–40
Chen C, Yang ST, Ahn WS, Ryoo R Chem Commun (2009) 3627–3629
Fauth DJ, Filburn TP, Gray ML, Hedges SW, Hoffman JS, Pennline HW, DOE/NETL-IR-2007-156
Liu SH, Wu CH, Lee HK, Liu SB (2010) Top Catal 53:210–217
Su F, Lu C, Kuo S-C, Zeng W (2010) Energy Fuel 24:1441–1448
Bhagiyalakshmi M, Yun LJ, Anuradha R, Jang HT (2010) J Hazard Mater 175:928–938
Fisher JC, Tanthana J, Chuang SSC (2009) Environ Prog Sustain Energy 28:589–598
Yue MB, Sun LB, Cao Y (2008) Microporous Mesoporous Mater 114:74–81
Yue MB, Chun Y, Cao Y (2006) Adv Funct Mater 16:1717–1722
Chong ASM, Zhao XS (2003) J Phys Chem B 107:12650–12657
Aguado J, Arsuaga JM, Arencibia A, Lindo M, Gascón V (2009) J Hazard Mater 163:213–221
Leal O, Bolívar C, Ovalles C, García JJ, Espidel Y (1995) Inorg Chim Acta 240:183–189
Huang HY, Yang RT (2003) Ind Eng Chem Res 42:2427–2433
Knowles GP, Graham JV, Delaney SW, Chaffee AL (2005) Fuel Process Technol 86:1435–1448
Knowles GP, Delaney SW, Chaffee AL (2005) Stud Surf Sci Catal 156:887–896
Knowles GP, Delaney SW, Chaffee AL (2006) Ind Eng Chem Res 45:2626–2633
Harlick PJE, Sayari A (2007) Ind Eng Chem Res 46:446–458
Van der Voort P, Gills-D’Hamers I, Vrancken KC, Vansant EF (1991) Faraday Trans 87:3899–3905
Drage TC, Blackman JM, Pevida C, Snape CE (2009) Energy Fuel 23:2790–2796
Cavenati S, Grande CA, Rodrigues AE (2004) J Chem Eng Data 19:1095–1101
Socrates G (2001) Infrared and Raman characteristic group frequencies. Wiley, UK
Ishikawa N, Kitazume T (1972) Chem Lett 169–170
Kimura M, Kuroda Y, Yamamoto O, Kubo M (1961) Bull Chem Soc Jpn 34:1081–1086
Lebel NA, Banucci E (1971) J Org Chem 36:2440–2448
Armor JN (1982) U.S. Patent 4.337.358
Armor JN, Zambri PM (1982) J Catal 73:57–65
Matsumura Y, Hashimoto K, Moffat JB (1992) J Phys Chem 96:10448–10449
Trejda M, Ziolek M, Decyk P, Duczmal D (2009) Microporous Mesoporous Mater 120:214–220
Xie Y, Sharma KK, Anan A, Wang G, Biradar AV, Asefa T (2009) J Catal 265:131–140
Khatri RA, Chuang SSC, Soong Y, Gray M (2006) Energy Fuel 20:1514–1520
Wei J, Shi J, Pan H, Su Q, Zhu J, Shi Y (2009) Microporous Mesoporous Mater 117:596–602
Acknowledgments
This study was carried out within the framework of the CENIT CO2 Project, supported by CDTI—Spanish Industry Department (www.cenitco2.es).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calleja, G., Sanz, R., Arencibia, A. et al. Influence of Drying Conditions on Amine-Functionalized SBA-15 as Adsorbent of CO2 . Top Catal 54, 135–145 (2011). https://doi.org/10.1007/s11244-011-9652-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-011-9652-7