Abstract
Three cobalt coordination polymers, namely {[Co(4-pmntd)2(CF3SO3)(H2O)]·(CF3SO3)·(C2H5OH)·0.5(HCCl3)·H2O} n (1), {[Co(4-pmntd)(H2O)4]·(NO3)2·H2O} n (2), and {[Co(4-pmntd)(pta)(CH3OH)2]·2(CHCl3)} n (3) (4-pmntd = N,N′-bis(4-pyridylmethyl)-naphthalene diimide; pta = terephthalate), have been synthesized by reactions of 4-pmntd with Co(CF3SO3)2·4H2O, Co(NO3)2·6H2O, and Co(pta), respectively. The complexes have been characterized by elemental analyses, powder X-ray diffraction, thermogravimetric analyses, IR spectroscopy, and single-crystal X-ray diffraction. In complex 1, the ligand adopts U-mode, resulting in a dumbbell-like 2D (4, 4)-sql net structure in a non-interpenetrating edge-to-edge arrangement. The Z-mode ligand arrangement leads to 1D zigzag chains for complex 2, and a 2D twofold interpenetrating bat-like (4, 4)-sql net for complex 3. A solution of complex 2 in DMF showed a change in color and remarkably enhanced fluorescence upon the addition of two equivalents of acetate, whereas other anions did not induce a color change. Hence, complex 2 can act as a highly promising acetate-detecting reagent. Their thermal and the gas adsorption properties of complexes 1 and 3 were investigated.
Graphical Abstract
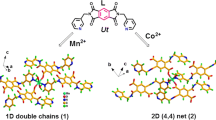
Three 1D and 2D cobalt coordination polymers have been synthesized; their structural diversity in these complexes is attributed to different coordination abilities, geometries of counter-anions, and the different conformations adopted by the ligand. A solution of complex 2 showed a change in color and remarkably enhanced fluorescence upon the addition of two equivalents of acetate, whereas other anions did not induce a color change. Hence complex 2 can act as a highly promising acetate-detecting reagent. Their thermal properties and the gas adsorption property of complexes 1, 3 were investigated.













Similar content being viewed by others
References
Coronado E, Espallargas GM (2013) Chem Soc Rev 42:1525
Robin AY, Fromm KM (2006) Coord Chem Rev 250:2127
Gao WY, Wojtas L, Ma SQ (2014) Chem Commun 50:5316
He HM, Sun FX, Jia JT, Bian Z, Zhao N, Qiu XP, Gao LX, Zhu GS (2014) Cryst Growth Des 14:4285
McMorran DA (2009) Inorg Chem 47:592
Fu YL, Xu ZW, Ren JL, Yang JY (2007) Cryst Growth Des 7:1198
Zang SQ, Su Y, Duan CY, Li YZ, Zhu HZ, Meng QJ (2006) Chem Commun 42:4997
Díaz P, Tovilla JA, Ballester P, Benet-Buchholz J, Vilar R (2007) Dalton Trans 32:3516
Amouri H, Desmarets C, Bettoschi A, Rager MN, Boubekeur K, Rabu P, Drillon M (2007) Chem Eur J 13:5401
Vickers MS, Beer PD (2007) Chem Soc Rev 36:211
Alfonso I, Bolte M, Bru M, Burguete MI, Luis SV, Rubio J (2008) J Am Chem Soc 130:6137
Liu ZM, Liu Y, Zheng SR, Yu ZQ, Pan M, Su CY (2007) Inorg Chem 46:814
Li GB, Liu JM, Cai YP, Su CY (2011) Cryst Growth Des 11:2763
Li GB, Liu JM, Yu ZQ, Wang W, Su CY (2009) Inorg Chem 49:8659
Li GB, Liu JM, Pan M, Su CY (2012) Dalton Trans 15:4626
Li GB, He JR, Liu JM, Su CY (2012) CrystEngComm 2152
Gale PA (2011) Anion receptor chemistry. Chem Commun 47:82
Gale P, Busschaert N, Haynes CJE, Karagiannidis LE, Kirby IL (2014) Chem Soc Rev 43:205
Wenzel M, Hiscock JR, Gale PA (2012) Chem Soc Rev 41:480
Cametti M, Rissanen K (2009) Chem Commun 45:2809
Madhuprasad AN, Shetty DR (2012) Trivedi. RSC Adv 2:10499
Murali MG, Vishnumurthy KA, Seethamraju S, Ramamurthy PC (2014) RSC Adv 4:20592
Sheldrick GM (1997) SHELXS-97, program for the solution of crystal structures. University of Göttingen, Germany
G. M. Sheldrick, SHELXL-97, Program for the Refinement of Crystal Structures, university of Göttingen, Germany, 1997
Zhao Y, Truhlar DG (2006) J Chem Phys 125:194101
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215
Dunning TH (1970) J Chem Phys 53:2823
Dunning TH, Hay PJ (1977) Methods of electronic structure theory. In: Schaefer HF III (ed.) Plenum, New York, pp 1–27
Miertus S, Scrocco E, Tomasi J (1981) Chem Phys 55:117
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21403191), the Natural Science Foundation of Guangdong Province (No. 2014A030307010), Zhanjiang Science Technology Project (No. 2013B01054), and Lingnan Normal University Science Research Foundation (No. ZL1309).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, GB., Liao, ZH., Pan, RK. et al. Synthesis, fluorescence, and sorption properties of cobalt coordination polymers of the N,N′-bis(4-pyridylmethyl)naphthalene diimide ligand. Transition Met Chem 40, 691–697 (2015). https://doi.org/10.1007/s11243-015-9963-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-015-9963-9