Abstract
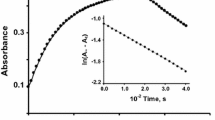
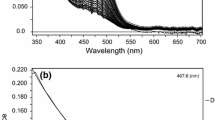
The redox reactions of thiosulfate with four iron(III) complexes having phenolate-amide-amine coordination, FeIII(L){L = 1,2-bis(2-hydroxybenzamido)ethane, L1; 1,3-bis(2-hydroxybenzamido)propane, L2; 1,5-bis(2-hydroxybenzamido)3-azapentane, L3; and 1,8-bis(2-hydroxybenzamido)3,6-diazaoctane, L4} have been investigated in 10% v/v MeOH + H2O and I = 0.3 mol dm−3. At constant pH (~ 4.8) and under pseudo-first order conditions of [S2O 2−3 ] the reaction obeyed the rate law : − d[FeIII(L)]/dt = k obs [FeIII(L)] + k ′obs where k ′obs denotes the observed rate constant of thiosulfate decomposition; k obs = a[S2O 2−3 ] + b[S2O 2−3 ] 2T is valid for all the complexes, particularly at pH < 6, while k ′obs = [H+][S2O 2−3 ] 2T is consistent with the rate law for thiosulfate decomposition proposed earlier. The rate data (k obs) were analysed on the basis of the reactivities of various species of FeIII(L) generated by the equilibrium protonation of the sec-NH of dien and trien spacer units resulting in the ring opening (for [FeIII(L3/L4)]), and acid base equilibrium of the aqua ligand bound to the iron(III) centre ([FeIII(L)(OH2) n ]). The redox activities, both for second and third order paths, show the ligand dependencies : L4<L3<L1<L2 conforming to the fact that the complexes tend to be less susceptible to electron transfer from S2O 2−3 with (i) the increase of the number of chelate rings, (ii) the decrease of overall charge, and (iii) the decrease of ring size offered by the amine moiety (from six membered to five membered one as for [FeIII(L1/L2)(OH2)2]+. There was no evidence for the formation of inner sphere thiosulfato complex, the possibility of the formation of the outer sphere ion-pairs, [Fe(L/HL)(OH2)n +/2+, S2O 2−3 ] with low equilibrium constant value may not be excluded. In view of this, the outer sphere electron transfer (ET) mechanism is the most likely possibility.
Similar content being viewed by others
References
R. Sarala D.M. Stanbury (1992) Inorg. Chem. 31 2771 Occurrence Handle1:CAS:528:DyaK38XksFKrs7g%3D Occurrence Handle10.1021/ic00039a021
(a) D.M. Stanbury, W.K. Wilmarth, S. Khalof, H.N. Po and J.E. Bryd, Inorg. Chem., 19, 2715 (1980); (b). W.K. Wilmarth, D.M. Stanbury, J.E. Bryd, H.N. Po and C.P. Chua, Coord. Chem. Rev., 51, 155 (1983)
B. Chaudhuri and R. Banerjee, J. Chem. Soc., Dalton Trans., 3451 (1998)
(a) F.M. Page, Trans. Faraday Soc., 50, 120 (1954); (b) G. Mahapatra, C. Nanda and D. Patnaik, J. Ind. Chem. Soc., 34, 457 (1957); (c) R. Schmid, Z. Phys. Chem., A 148, 321 (1930)
(a) A.C. Dash, A.K. Patnaik, and N. Das, Ind. J. Chem., 36A, 268 (1997); (b) A.C. Dash and K.C. Jena, Transition. Met. Chem., 22, 141 (1997); (c) A. Das and A.C. Dash, J. Chem. Soc., Dalton Trans., 1949 (2000); (d) S. Nayak and A.C. Dash, Ind. J. Chem., 42A, 2427 (2003); (e) A. Das and A.C. Dash, Ind. J. Chem., 39A, 902 (2000); (f) S. Nayak, A.C. Dash, P.K. Nayak and D. Das, Transition. Met. Chem., 30, 917 (2005)
H. Ojima (1967) Nippon Kagaku Kaishi 88 329 Occurrence Handle1:CAS:528:DyaF2sXktVOmurc%3D
C.R. Dennis J.G. Leipoldt S.S. Basson G.J. Lamprecht (1985) Polyhedron 4 1621 Occurrence Handle1:CAS:528:DyaL2MXlvVCntr8%3D Occurrence Handle10.1016/S0277-5387(00)87238-1
R. Sarala S.B. Rabin D.M. Stanbury (1991) Inorg Chem. 30 3999 Occurrence Handle1:CAS:528:DyaK3MXlvFSku7w%3D Occurrence Handle10.1021/ic00021a007
A.E. Harvey SuffixJr. J.A. Smart E.S. Amis (1955) Anal. Chem. 27 26 Occurrence Handle1:CAS:528:DyaG2MXislaqsQ%3D%3D Occurrence Handle10.1021/ac60097a009
J.J. Byerley, S.A. Fouda and G.L. Rampel, J. Chem. Soc. Dalton Trans., 889 (1973)
A.I. Vogel (1942) Qualitative Chemical Analysis Longmans Green and Co. London 329
R.M. Smith and A.E. Martell (Eds.), Crtical Stability Constants, Plenum, New York, 1976, p. 86
(a) R. Mehnert and O. Brede, Radiat. Phys. Chem., 23, 463 (1984); (b) M. Schoneshofer, Int. J. Radiat. Phys. Chem., 5, 375 (1973); (c) R. Mehnert, O. Brede and I. Janovsky, Radiochem. Radioanal. Lett. 53, 299 (1982)
S. Nayak and A.C. Dash, Transition. Met. Chem., 31, 813 (2006)
D.E. Pennington, in A. E. Martell (Ed.), Coordination Chemistry, ACS Monograph 174, Vol, 2, American Chemical Society, Washington DC, 1978, p. 487, Table 3-2
M. Ali S.K. Saha P. Banerjee (1990) Ind. J. Chem. 29 528
K.K. Sengupta S. Das S. Sen Gupta (1988) Transition. Met. Chem. 13 155 Occurrence Handle1:CAS:528:DyaL1MXksFOmug%3D%3D Occurrence Handle10.1007/BF01087810
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nayak, S., Dash, A.C. Oxidation of the thiosulfate ion by some iron(III) complexes with phenolate - amide-amine coordination: A comparative kinetic study. Transition Met Chem 31, 930–937 (2006). https://doi.org/10.1007/s11243-006-0086-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11243-006-0086-1