Abstract
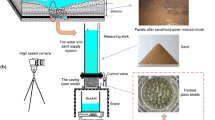
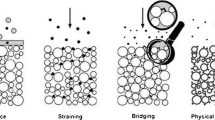
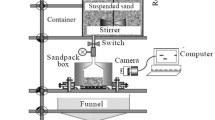
This study investigated the effect of flow velocity, the concentration of red mud particles, and the concentration of \(\hbox {OH}^{-}\) ions on the penetration processes of red mud filtrate with fine particles in a porous medium by seepage. The results show that the peak concentrations of the breakthrough curves (BTCs) of red mud particles with high alkalinity are much higher than that with low alkalinity, indicating that the existence of \(\hbox {OH}^{-}\) ions enhances the repulsive interaction between red mud particles and between red mud particles and the matrix and promotes the migration of red mud particles. The red mud particles are more easily absorb onto the surface of porous medium or embedded in the matrix due to the greater adsorption between red mud particles and porous dielectric matrix than silicon powders. The penetration velocity of these red mud particles is often slower than water velocity due to the capture effect by straining and the detours path effect, especially in the case of high injection concentration and low alkalinity. Both the recovery rate and modal size of recovered particles increase with the increase in flow velocity, and the recovery rate of particles with high alkalinity is higher than that of particles with low alkalinity, which can be attributed to the stronger repulsive interaction between particles and between particles and the matrix. An analytical solution for the migration of particles in a porous medium in which the contaminant intensity varies with time has been developed from the elementary solution, and the predicted BTCs for a repeated three-pulse injection are in good accordance with the experimental results.












Similar content being viewed by others
Abbreviations
- \(\hbox {C}_{{({\mathrm{OH}}^{-})}}\) :
-
Concentration of \(\hbox {OH}^{-}\) ions
- d :
-
Particle size
- \(D_{50}\) :
-
Median grain diameter
- \(C_{\mathrm{c}}\) :
-
Coefficient of curvature
- \(C_{\mathrm{u}}\) :
-
Uniformity index
- n :
-
Porosity of the porous medium
- \(C_{{\mathrm{inj}}}\) :
-
Concentration of injected particles
- v :
-
Darcy velocity
- C :
-
Concentration of suspended particles
- N :
-
Turbidity level
- a, b, and c :
-
Fitting parameters
- \(R^{2}\) :
-
Coefficient of determination
- z :
-
Water flow direction
- D :
-
Hydrodynamic dispersion coefficient
- u :
-
Average interstitial particle velocity
- t :
-
Time
- \(\sigma \) :
-
Concentration of particles deposited onto the solid matrix
- \(\rho _{\mathrm{s}}\) :
-
Bulk density of the solid matrix
- \(k_{\mathrm{d}}\) :
-
Deposition coefficient
- \(k_{\mathrm{r}}\) :
-
Release coefficient
- s, r :
-
Laplace transform variables of t and z
- \(L^{-1}\) :
-
Laplace inverse operator
- \(\alpha \) :
-
Arbitrary constant
- \(I_{0}, I_{1}\) :
-
Modified Bessel function of the first kind of order zero and one
- \(\alpha _{1},\alpha _{2}\) :
-
Arbitrary constants
- \(\tau \) :
-
Dummy integration variable
- I :
-
Strength of plane source
- m :
-
Mass of injected particles
- A :
-
Cross-sectional area of column
- \(\updelta (\cdot )\) :
-
Dirac delta function
- \(t^\prime \) :
-
Particle injection moment
- PV:
-
Pore volume number
- \(V_{\mathrm{inj}}\) :
-
Volume of particle liquid in each injection
- Q :
-
Flow rate of water
- \(t_{0}\) :
-
Duration time applied on the top surface of column
- \(t_{\mathrm{inj}}\) :
-
Injection time period of particles
- \(C_{\mathrm{out}}\) :
-
Particle concentration at the outlet
- \(C_{\mathrm{R}}\) :
-
Relative concentration
- \(V_{\mathrm{P}}\) :
-
Pore volume of the entire soil column
- \(\alpha _{\mathrm{d}}\) :
-
Dispersivity
- \(u_{0}\) :
-
Average interstitial fluid velocity
- \(\kappa \) :
-
Exponent of power law
- \(R_{\mathrm{e}}\) :
-
Recovery rate
References
Ahfir, N.D., Benamar, A., Alem, A., Wang, H.Q.: Influence of internal structure and medium length on transport and deposition of suspended particles: a laboratory study. Transp. Porous Media 76(2), 289–307 (2009)
Auset, M., Keller, A.A.: Pore-scale processes that control dispersion of colloids in saturated porous media. Water Resour. Res. 40(3), 1–11 (2004)
Bai, B.: Response of saturated porous media subjected to local thermal loading on the surface of semi-infinite space. Acta Mech. Sin. 22(1), 54–61 (2006)
Bedrikovetsky, P., Zeinijahromi, A., Siqueira, F., Furtado, C., Souza, A.L.: Particle detachment under velocity alternation during suspension transport in porous media. Transp. Porous Media 91(1), 173–197 (2012)
Bekhit, H.M., EI-Kordy, M.A., Hassan, A.E.: Contaminant transport in groundwater in the presence of colloids and bacteria: model development and verification. J. Contam. Hydrol. 108, 152–167 (2009)
Bradford, S.A., Yates, S.R., Bettahar, M., Simunek, J.: Physical factors affecting the transport and fate of colloids in saturated porous media. Water Resour. Res. 38(12), 1327–1338 (2002)
Chang, C.M., Wang, M.K., Chang, T.W., Lin, C.: Transport modeling of copper and cadmium with linear and nonlinear retardation factors. Chemosphere 43(8), 1133–1139 (2001)
Christian, J., David, S., Stephen, F.: Coupled multi-ion electrodiffusion analysis for clay soils. Can. Geotech. J. 41(2), 287–298 (2004)
Fesch, C., Simon, W., Haderlein, S.B., Reichert, P., Schwarzenbach, R.P.: Nonlinear sorption and nonequilibrium solute transport in aggregated porous media: experiments, process identification and modeling. J. Contam. Hydrol. 31(3–4), 373–407 (1998)
James, S.C., Chrysikopoulos, C.V.: Analytical solutions for monodisperse and polydisperse colloid transport in uniform fractures. Colloids Surf. Physicochem. Eng. Asp. 226(1–3), 101–118 (2003)
Katzourakis, V.E., Chrysikopoulos, C.V.: Mathematical modeling of colloid and virus cotransport in porous media: application to experimental data. Adv. Water Resour. 68, 62–73 (2014)
Kim, H.N., Walker, S.L.: Escherichia coli transport in porous media: influence of cell strain, solution chemistry, and temperature. Colloids Surf. B Biointerfaces 71(1), 160–167 (2009)
Klauber, C., Grafe, M., Power, G.: Bauxite residue issues: II. Options for residue utilization. Hydrometallurgy 108, 11–32 (2011)
Knight, M.A., Mitchell, R.J.: Modeling of light non-aqueous phase liquid release into unsaturated sand. J. Contam. Hydrol. 20, 1–25 (1995)
Kretzschmar, R., Barmettler, K., Grolimund, D., Yan, Y.D., borkovec, M., Sticher, H.: Experimental determination of colloid deposition rates and collision efficiencies in natural porous media. Water Resour. Res. 33(5), 1129–1137 (1997)
Kumar, A., Kumar, S.: Development of paving blocks from synergistic use of red mud and fly ash using geopolymerization. Constr. Build. Mater. 38, 865–871 (2013)
Massei, N., Lacroix, M., Wang, H.Q., Dupont, J.P.: Transport of particulate material and dissolved tracer in a highly permeable porous medium: comparison of the transfer parameters. J. Contam. Hydrol. 57(1–2), 21–39 (2002)
Patrick, J., Fox, J.L.: Model for consolidation-induced solute transport with nonlinear and nonequilibrium sorption. Int. J. Numer. Anal. Methods Geotech. 8(3), 188–198 (2008)
Piga, F.P., Stoppa, L.: Recovering metals from red mud generated during alumina production. JOM 45(11), 55–59 (1993)
Porubcan, A.A., Xu, S.P.: Colloid straining within saturated heterogeneous porous media. Water Resour. Res. 45(4), 1796–1806 (2011)
Sandra, G., Susanna, W., Mats, J.: Effects of temperature on the stability of colloidal montmorillonite particles at different pH and ionic strength. Appl. Clay Sci. 43, 21–26 (2009)
Senff, L., Hotza, D., Labrincha, J.A.: Effect of red mud addition on the rheological behavior and on hardened state characteristics of cement mortars. Constr. Build. Mater. 25(1), 163–170 (2011)
Touch, N., Nakashita, S., Hibino, T.: Deposition behavior of mud in sand beds under the effects of organic properties. Transp. Porous Media 91(2), 531–546 (2012)
Wang, H.Q., Lacroix, M., Massei, N., Dupont, J.P.: Particle transport in porous medium: determination of hydrodispersive characteristics and deposition rates. C. R. Acad. Sci. Earth Planet. Sci. 331(2), 97–104 (2000)
Wang, J., Hu, J., Zhang, S.: Studies on the sorption of tetracycline onto clays and marine sediment from seawater. J. Colloid Interface Sci. 349(2), 578–582 (2010)
Wu, G., Li, L.Y.: Modeling of heavy metal migration in sand/bentonite and the leachate pH effect. J. Contam. Hydrol. 33, 313–336 (1998)
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (51678043; 51478034) and National Key Basic Research Program of China (2015CB057800), to which the authors are very grateful.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bai, B., Wang, J., Zhai, Z. et al. The Penetration Processes of Red Mud Filtrate in a Porous Medium by Seepage. Transp Porous Med 117, 207–227 (2017). https://doi.org/10.1007/s11242-017-0829-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-017-0829-9