Abstract
Cultures of Nasturtium officinale were cultivated in vitro under illumination with different wavelengths of light-emitting diode (LED) light (white LED light—WLED, blue light—B, red light—R, 70% red and 30% blue light—RB, 50% green, 35% red and 15% blue light—RBG, 50% yellow, 35% red and 15% blue light—RBY, 50% far red, 35% red and 15% blue light—RBfR, 50% UV, 35% red and 15% blue light—RBUV, darkness—D), and under white fluorescent light (WF)—control conditions. The study investigated the influence of the applied lighting conditions on biomass growth and the production of glucosinolates, phenolic compounds, as well as photosynthetic pigments, and soluble sugars. The study showed a significant beneficial effect of the RBG light on biomass growth (Gi = 11.81 after 20 days) and the production of glucosinolates. The total glucosinolate content under these conditions increased 5.8 and 1.4 times in comparison with the WF light and D condition, respectively, reaching 237.92 mg 100 g−1 DW. The production of phenolic compounds, sugars, and photosynthetic pigments was comparable to the production under the control conditions. The antioxidant potential of extracts from the cultivated biomass was assessed by the CUPRAC, DPPH, and FRAP assays. Extracts obtained from the biomass of cultures grown under the RBG light had an antioxidant potential similar to that of the control cultures. This is the first report providing evidence of the stimulating effect of light quality on the biomass yield and production of glucosinolates by N. officinale microshoot cultures in vitro.
Key message
The studies of different LED light quality on Nasturtium officinale microshoot cultures were proven that combination of 50% green, 35% red and 15% blue lights stimulated the production of glucosinolates, phenolic compounds, photosynthetic pigments, and influenced on the antioxidant potential of biomass extracts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant biotechnology strategies may be used to manipulate the production of valuable secondary metabolites responsible for the biological activity of different plant species. One of the advantages is their use in research on rare and protected plants. The main factors affecting the production of secondary metabolites are basal medium composition, quality and quantity of plant growth regulators (PGRs), and lighting conditions (Mulabagal and Tsay 2004; Karuppusamy 2009).
Different intensities of light, different wavelengths, and photoperiods, and also lack of light could be used as the physical conditions to influence the production of valuable products in plant in vitro cultures (Ramawat and Mathur 2007; Ramakrishna and Ravishankar 2011). Studies have shown that light can stimulate the production of different groups of secondary metabolites such as anthocyanins (Zhong et al. 1991), polyamines (Shin et al. 2004), polyphenols (Szopa et al. 2018), phenylpropanoids (Kubica et al. 2020), and also other biologically active compounds, e.g. artemisinin, digoxin, taxol, or vinblastine (Ramakrishna and Ravishankar 2011).
The object of our research was Nasturtium officinale R. Br. (Robert Brown; described earlier by Linnaeus as Sisymbrium nasturtium-aquaticum), from the family Brassicaceae, known as watercress. It is a rare perennial, aquatic or semi-aquatic herb with characteristic creeping or floating stems, native to Europe, North Africa, and Asia (Klimek-Szczykutowicz et al. 2018). The Red List of Threatened Species of the International Union for Conservation of Nature has classified this species as a plant of least concern in Europe (IUCN).
Nasturtium officinale is a species known well the from food industry but is also used in phytopharmacology and cosmetology. It has scientifically proven biological activities, such as anticancer, antioxidant, antimicrobial, and anti-inflammatory properties (Bahramikia and Yazdanparast 2010; Yehuda et al. 2012; Casanova et al. 2013; Sadeghi et al. 2014). They are determined by a wide range of compounds found in the N. officinale plant, e.g. glucosinolates, isothiocyanates, polyphenols (flavonoids, phenolic acids, proanthocyanidins), carotenoids, vitamins, and bioelements (Afsharypuor and Salehi 2008; Boligon et al. 2013; Jeon et al. 2017). N. officinale plants recently have been the subject of genetic research relating to the identification of genes associated with enzymes associated with the phenylpropanoid biosynthetic pathway. During the research, 11 genes coding of enzymes in the leaves, stems, roots, flowers, and seeds of N. officinale were identified. HPLC analysis showed the presence of seven phenolic compounds (chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, rutoside, quercetin, and kaempferol) as well as their positive correlation between gene expression and the accumulation of these compounds (Bong et al. 2020).
In vitro cultures of N. officinale have not been the subject of many biotechnological studies. Those reported were mainly studies on micropropagation (Wainwright and Marsh 1986) and genetic transformation by Agrobacterium rhizogenes (Wielanek et al. 2009; Park et al. 2011). Our studies on the accumulation of bioelements, secondary metabolites, and the biological activity of biomass extracts have recently been published (Klimek-Szczykutowicz et al. 2019, 2020a, b).
The present research aimed to investigate the influence of LED light quality on the accumulation of metabolites: glucosinolates, phenolic compounds, soluble sugars, and photosynthetic pigments, as well as on the antioxidant potential of N. officinale microshoot extracts.
Materials and methods
Experimental in vitro cultures
Initial N. officinale microshoot cultures were established as reported previously by us (Klimek-Szczykutowicz et al. 2019). Based on our earlier studies, we used the optimal medium for the best “growth” and “production” in terms of the accumulation of glucosinolates, phenolic compounds, and antioxidant activity (Klimek-Szczykutowicz et al. 2020b). The chosen medium was the Murashige and Skoog (MS) medium (Murashige and Skoog 1962) supplemented with 1 mg L−1 6-benzyladenine (BA) and 1 mg L−1 1-naphthaleneacetic acid (NAA), 30 g L−1 sucrose, and 7.2 g agar. The experimental cultures were maintained over 20-day growth periods (3 series), in Magenta™ vessels (Sigma-Aldrich, product No. V8630, Poznań, Poland); medium volume was set at 50 mL, inoculum amount was 1 g FW (fresh weight). The cultures were maintained under a 16/8 h photoperiod, at 25/23 ± 1 °C (day/night), and at 70% relative humidity in a phytotron. All the experiments and measurements were performed in triplicate (including reagent blanks).
In the experiment, eight variants of illumination with light-emitting diode (LED) lights (combinations of different wavelengths) were tested: blue light—B (λ = 430 nm), red light—R (λ = 670 nm), a combination of 70% red and 30% blue light—RB, a combination of 50% UV-A (λ = 400 nm), 35% red and 15% blue light—RBUV, a combination of 50% far red (λ = 730 nm), 35% red and 15% blue light—RBfR, a combination of 50% green (λ = 528 nm), 35% red and 15% blue light—RBG, a combination of 50% yellow (λ = 600, 630 nm), 35% red and 15% blue light—RBY, and white LED light—WLED (33% warm—2700 K, 33% neutral—4500 K, 33% cold—5700 K). The control conditions were established with white fluorescent lamps—WF (Philips TL-D 36W/54 cool fluorescent lamps) (λ = 390–760 nm), and with lack of light—D (darkness). In total, ten different lighting conditions were tested. The PPFD was 40 µmol m−2 s−1 in all the combinations.
Calculation of growth index
Biomass increments were expressed by the growth index (Gi). Dried weight (DW) was measured for each sample after lyophylization (Labconco Cooperation, Kansas City, MO, USA). The Gi was calculated using the formula: Gi = \(\frac{{DW}_{1}-{DW}_{0}}{{DW}_{0}}\), where Dw1—dry weight of microshoots at the end of the experiment, and Dw0—dry weight of the inoculum (Grzegorczyk and Wysokińska 2008).
Sample preparation
Plant material harvested from the tested N. officinale microshoot cultures was immediately frozen in liquid N2 and lyophilized (freeze dryer, Labconco Corporation, Kansas City, MO, USA). The dry biomass was pulverized (MM400, Retch, Haan, Germany). Samples (0.2 g) were weighed out and extracted twice with 4 mL of methanol (STANLAB, Lublin, Poland) under sonication for 20 min. at 25 °C (ultrasonic bath; POLSONIC 2, frequency 40 kHz, Warsaw, Poland). Then the samples were centrifuged (7 min., 2000×g; MPW-223E centrifuge; MPW, Warsaw, Poland) and filtered (0.22 μm syringe filters; Millex®GP; Merck Millipore, Burlington, MA, USA). If not otherwise stated, these extracts were used for further analyses.
Spectrophotometric analysis of the total GSL pool
Total amounts of glucosinolates were determined according to Gallaher et al. (Gallaher et al. 2012), as reported previously (Klimek-Szczykutowicz et al. 2020a, b), based on their alkaline hydrolysis and subsequent reaction of released 1-thioglucose with ferricyanide. A decrease in absorbance was read at 420 nm (Synergy II, BioTek, Winooski, VT, USA). Briefly, lyophilized and pulverized samples were extracted after myrosinase inactivation. The centrifuged extract was evaporated under N2 (TurboVap evaporator, Zymark, Midland, MI, USA), and after reconstitution in H2O the samples were subjected to anion-exchange SPE (Supel-Select SAX, 60 mg, 3 mL, Bellefonte, PA, USA) to clean up. The GSLs were eluted with 4 × 0.25 mL of 0.5 M NaCl then dried under N2, and the GSLs were hydrolysed with 1 M sodium hydroxide. After 30 min., the samples were neutralised with concentrated hydrochloric acid. The colorimetric reaction of 2 mM potassium ferricyanide solution (0.4 M phosphate buffer pH 7.0) with the standard or sample solution was monitored in 96-well plates. Absorbance was read at 420 nm (Synergy II, BioTek, Winooski, VT, USA) 2 min. after ferricyanide addition. The total GSL content was expressed as sinigrin equivalent in mg of sinigrin (SIN)/100 g DW.
Analysis of total flavonoids
Total flavonoid content was estimated according to the method described by Ramos et al. (2017). Forty microliters of 10% AlCl3 was mixed with 100 μL of methanolic extract and samples were filled to 1000 μL with 5% acetic acid. The absorbance of Al3+ flavonoid complex was recorded at 425 nm in a 96-well plate format (Synergy II). The sum of flavonoids was calculated as mg of rutoside equivalent (RE)/100 g DW. The measurements were done in triplicate (including reagent blanks).
Analysis of total polyphenols
Total phenolic content was measured according to Singleton et al. (Singleton et al. 1999), with the Folin–Ciocalteu (F–C) reagent, as previously reported (Klimek-Szczykutowicz et al. 2020a, b). The methanolic extracts were prepared as described in Sect. 2.3. Water-diluted F–C phenol reagent (5/2 v/v, 0.45 mL) was mixed with the sampled extracts (100 μL). After 10 min., saturated Na2CO3 (0.45 mL) was added. The incubation of the samples was continued for 2 h in darkness at 25 °C. Then, the samples were centrifuged and aliquoted to 96-well plates. The absorbance was detected at 760 nm (Synergy II, BioTek, Winooski, VT, USA). The pool of phenolic compounds was calculated as mg gallic acid (GAL)/100 g DW. The analysis was done in triplicate (including reagent blanks).
Analysis of total soluble saccharide
For analyzing soluble sugars, the phenol–sulfuric method (Dubois et al. 1951) modified by Bach et al. (2015) was used. Samples of water extract were mixed with 5% phenol solution and concentrated sulfuric acid. Absorbance was measured at 490 nm in a 96-well plate format (Synergy II, BioTek, Winooski, VT, USA). The saccharide content was expressed as glucose (GLU) equivalents.
Determination of photosynthetic pigments
Photosynthetic pigments (chlorophyll a and b, and carotenoids) were determined spectrophotometrically using a UV/VIS spectrophotometer (Helios Alpha, Unicam Ltd., Cambridge, GB). Material was homogenized in a mortar with the addition of a small amount of calcium carbonate, quartz sand, and 80% acetone (15 mL) added in portions. After filtration, the solution was made up to 20 mL with acetone. Absorbance was read at 663.2 nm for chlorophyll a and 646.8 nm for chlorophyll b, and 470 nm for carotenoids (Lichtenthaler and Buschmann 2001).
Assays of antioxidant potential
CUPRAC assay
To determine the total antioxidant activity, the CUPric Reducing Antioxidant Capacity (CUPRAC) method (Özyürek et al. 2007) modified as reported previously (Biesaga-Kościelniak et al. 2014, and Klimek-Szczykutowicz et al. 2020a, b) was used. Equal volumes of methanolic extracts (prepared as described in Sect. 2.3), 10 mmol/L Cu2+, 7.5 mmol/L neocuproine, and 1 mol/L ammonia-acetate buffer (pH 7.0) were sequentially dispensed. The samples were mixed and incubated for 15 min. (25 °C). The reduction of the neocuproine-Cu2+ complex was monitored spectrophotometrically at 425 nm in a 96-well plate format (Synergy II, BioTek, Winooski, VT, USA). The antioxidants accumulation was calculated as mmol Trolox equivalent (TE)/100 g DW. The measurements were done in triplicate (including reagent blanks).
DPPH radical-scavenging activity assay
The antioxidant potential of the extracts was also estimated using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) (Blios 1958). Fifty microliters of extracts were mixed with 150 μL of DPPH methanolic solution. The samples were incubated for 60 min., and then their absorbance was read at 517 nm (96-well plates, Synergy II, BioTek, Winooski, VT, USA). The measurements were performed in triplicate (including reagent blanks).
FRAP assay
The antioxidant capacity of the biomass methanolic extracts was also estimated employing the ferric reducing ability of plasma (FRAP) tests (Benzie and Strain 1996). The reagent consisted of a 10 mmol/L TPTZ (2,4,6-tris(2-pyridyl)-s-triazine) soluted in 40 mmol/L HCl with addition of FeCl3·6H2O (20 mmol/L) and 300 mmol/L of a pH 3.6 acetate buffer (1:1:10 v/v/v). Fifty microliters of extracts was poured with 150 μL of the prepared reagent. After 5 min incubation the absorbance of the sample was read at 593 nm (96-well plates, Synergy II, BioTek, Winooski, VT, USA). The measurements were done in triplicate (including reagent blanks).
Statistical analysis
The influence of light conditions was evaluated by one-way ANOVA. Differences between means were calculated using Duncan’s multiple range test (p < 0.05) using the statistical package STATISTICA 13.0 (Stat-Soft, Inc., Tulsa, OK, USA). The values show the means ± SD (standard deviation).
Results and discussion
Microshoot appearance and biomass growth
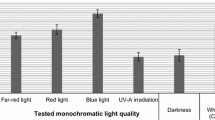
As a result of the experiment, different growth of microshoot culture biomass was observed after 20-day growth cycles, depending on the LED lighting conditions. In vitro cultures that were characterized by large amounts of microshoots and axillary shoots, and dark green colour were those under the B, RB, RBY, RBUV, and RBG light variants (Fig. 1). The unchanged appearance was also observed in the WF control and WLED light variant. Microshoots grown under the R, RBfR, and D light variants had a light green colour and their amounts were the smallest. The values of Gi ranged from 2.52 to 13.38 (Fig. 2). The highest Gi values were obtained for the microshoots grown under RBY and RBG lights—13.38 and 11.81, respectively. The lowest Gi values were obtained for the microshoots grown in the control, and in D 2.52. A clear stimulating effect of all the tested LED light variants on biomass increase was observed in the tested cultures, in comparison with the control conditions (Gi increases of up to 5.3 times). In our previous study, Gi was equal to 10.24 after 20 days of growth under continuous LED white light in microshoot agar cultures of N. officinale, and was similar to the growth increments obtained under this study (Klimek-Szczykutowicz et al. 2020b).
The appearance of N. officinale microshoot cultures depending on the applied LED lighting conditions [WF (Philips TL-D 36 W/54 cool fluorescent lamps) (λ = 390–760 nm), D darkness, WLED white LED light (33% warm—2700 K, 33% neutral—4500 K, 33% cold—5700 K), B blue light (λ = 430 nm), R red light (λ = 670 nm), a combination of 70% red and 30% blue light—RB, a combination of 50% green (λ = 528 nm), 35% red and 15% blue light—RBG, a combination of 50% yellow (λ = 600, 630 nm), 35% red and 15% blue light—RBY, a combination of 50% far red (λ = 730 nm), 35% red and 15% blue light—RBfR and a combination of 50% UV-A (λ = 400 nm), 35% red and 15% blue light—RBUV). Scale bar denotes 10 mm
Biomass increments expressed as a growth index (Gi) (calculated according to the formula: Gi = \(\frac{{DW}_{1}-{DW}_{0}}{{DW}_{0}}\); DW1—dry weight of microshoots and Dw0—dry weight of the inoculum) of N. officinale microshoot cultures depending on the applied LED lighting conditions. Data expressed as the mean value ± SD (n = 3). Different superscript letters (a–c) indicate significant differences between means (Duncan’s multiple range test; p < 0.05)
The total glucosinolate content
In this experiment, we also showed a significant impact of the eight tested LED light combinations on the production of selected groups of metabolites (Table 1). The total glucosinolate content ranged from 40.86 to 237.92 mg SIN 100 g−1 DW. The highest content was obtained for N. officinale microshoot cultures grown under the RBG light, and it was 5.8 times higher than under the WF (40.86 mg SIN 100 g−1 DW). Content higher than under the WF light was obtained in all tested light conditions except R (81.63 mg SIN 100 g−1 DW). The lowest glucosinolate content (40.86 mg SIN 100 g−1 DW) was obtained under the WF light, which is evidence that LED light tends to promote glucosinolate production (Table 1).
In our earlier study (Klimek-Szczykutowicz et al. 2020b), we had performed optimization of agar and agitated N. officinale microshoot cultures under continuous white LED light. For the cultures which had been grown over 20-day periods on MS medium with 1 mg L−1 BA and 1 mg L−1 NAA, the total glucosinolate content was 164.97 and 177.34 mg SIN 100 g−1 DW, respectively. The maximum total amount of glucosinolates under the RBG light was respectively 1.4 and 1.3 times higher, while for the WLED light 1.2 and 1.3 times lower, in comparison with those agar and agitated cultures.
Our studies are innovative concerning the influence of different LED lighting conditions on glucosinolate production in N. officinale microshoot cultures. A study by Choi et al. (Choi et al. 2020) had tested the influence of red, blue, and green lights at different proportions on the production of glucosinolates in N. officinale plants grown in a hydroponic system over 2 weeks. The highest content was obtained for mixed red and blue light (70:30%) and mixed red, blue and green light (33:33:33%). The study had shown a positive effect of combined red, blue, and green light on the production of glucosinolates, which was also confirmed in our studies.
Much earlier, Engelen-Eigles et al. (Engelen-Eigles et al. 2006) had performed a study concentrated on the effects of temperature, photoperiod, and light quality on gluconasturtiin production in N. officinale plants growing in vivo in special growth chambers. The tested light was emitted by metal halide lamps enriched with red or far-red lights, with the metal halide lamps alone used as the control. The authors had reported the highest gluconasturtiin content for plants grown under red light over 1 week (1074.4 mg 100 g−1 DW), with that content being 1.3 times higher than in the control (under metal halide lamps only). In our study, the total glucosinolate content in extracts from the biomass cultured under the R light was 2.0 times higher and 1.7 times lower than under the WF and WLED lights, respectively (Table 1).
Microgreens of the Brassica genus have also been the object of studies on the influence of different lighting conditions on the bioelement and vitamin content (Kamal et al. 2020). The studies confirmed the positive effects of a combination of red, green, and blue light (70:10:20%) on the growth and nutritional content of e.g. Brassica oleracea var. capitata f. rubra, B. oleracea var. italica, and B. oleracea var. capitata.
The total flavonoid and polyphenol content
The total flavonoid content in extracts from N. officinale microshoots cultured under the tested LED light variants ranged from 546.79 to 1149.45 mg RE 100 g−1 DW (Table 1). The highest total flavonoid content was recorded under the RBY light, and it was 1.4 times higher than under the WLED (842.82 mg RE 100 g−1 DW) light. A high amount was also obtained for the RBG light (1074.13 mg RE 100 g−1 DW), which was comparable to that obtained under the WF (1070.63 mg RE 100 g−1 DW) light and 1.3 times higher than under the WLED light. The lowest flavonoid content was obtained for the D condition, under which it was 2.0 and 1.5 times lower than under the WF and WLED lights, respectively (Table 1).
In an earlier study, we had determined total amounts of flavonoids in extracts from N. officinale microshoot cultures grown in a temporary immersion system (in a RITA® bioreactor), on MS medium containing 1 mg L−1 BA and 1 mg L−1 NAA, cultured over 20-day growth periods (Klimek-Szczykutowicz et al. 2020a). The results obtained for the bioreactor cultures (1171.59 mg RE 100 g−1 DW) were comparable to those reached by the microshoots cultured under the RBY light.
The F–C assay was used to evaluate the total polyphenol content in the studied cultures. The total polyphenol content varied from 190.84 to 229.18 mg GAL 100 g−1 DW (Table 1). The highest content was obtained under the WLED light, but similar results were also obtained for the WF (226.20 mg GAL 100 g−1 DW) and RBUV (224.84 mg GAL 100 g−1 DW) lights, and also the D condition (224.63 mg GAL 100 g−1 DW). The maximum amounts of total polyphenols in extracts from the biomass cultured under different lighting conditions were lower than those obtained in our former studies. The total polyphenol content for N. officinale agar and bioreactor cultures grown on MS medium with 1 mg L−1 BA and 1 mg L−1 NAA over 20-day growth periods under continuous LED white light were respectively 2.5 and 2.2 times higher than the maximum content reached in this study (Klimek-Szczykutowicz et al. 2020a, b) (Table 1).
The different LED lights used did not promote the production of phenolic compounds. There are other known studies in which the production of phenolic compounds did not increase by stimulation with different lighting conditions. Da Silva et al. (da Silva et al. 2020) had tested the influence of red and blue LED light (70:30%) on the total phenolic content in Moringa oleifera shoots cultures. In vitro cultures grown under white light (control) accumulated more phenolic compounds than under the red and blue light (70:30%) conditions. Moreover, a study on callus cultures of Ocimum basilicum had also reported a higher accumulation of chicoric acid under white LED light in comparison with red, blue, and green lights (Nadeem et al. 2019). Results of another study on callus cultures of Lepidium sativum (Ullah et al. 2019) also showed higher production of total phenolics and flavonoids under monochromatic white light than under red, green, blue, and yellow lights.
The total saccharides content
The analysis of the total amounts of soluble sugars in extracts from N. officinale microshoot cultures indicated their highest content under the WLED light (9.26 g GLU 100 g−1 DW). The lowest amounts of sugars were obtained in the in vitro cultures grown under the RBY (5.62 g GLU 100 g−1 DW) and B (5.69 g GLU 100 g−1 DW) lights (Table 1).
Photosynthetic pigments content
The quality of light had an impact on the physiological responses of plants, which was reflected in the level of photosynthetic pigments in the analysed biomasses (Table 2). N. officinale biomass cultivated in D had a level of pigments that was about 4 times lower than the highest values obtained. The highest chlorophyll a content was found in the biomass grown in the presence of B and RB light, and also RBY, RBUV and WLED light. Then the amount of this pigment was almost twice as high as that under the control WF light and also under the R light (Table 2). Contrary to the results of our research, a higher chlorophyll a content had been obtained in Solanum tuberosum cultures when the green light was added to red and blue LED light, but the addition of yellow light had no such effect (Li et al. 2018).
In the case of chlorophyll b, the greatest amount of this pigment was found in microshoots under the B light, which was twice as high as under the WF control conditions (Table 2).
A similar effect of blue light was observed on the carotenoid content, but the same level of carotenoids was confirmed in the biomass grown in the presence of the RB, RBY and RBUV light (Table 2). The results of studies on callus cultures of Verbena officinalis had confirmed the stimulation of the production of chlorophyll a and b and carotenoids by the RB light, but not under B or R light (Kubica et al. 2020). Additionally, the in vitro cultures of N. officinalis growing under the RB light produced 37.8, 18.2, and 22.9 times more chlorophylls a and b, and carotenoids, respectively, than the in vitro cultures of V. officinalis. Our studies can’t confirm the results of experiments conducted on in vitro cultures of Achillea millefolium, where the amounts of chlorophyll a and b, and carotenoids were influenced by the stimulating effect of green LED light (Alvarenga et al. 2015). In the N. officinale culture, the addition of the far red (fR) spectrum to the RB spectrum did not reduce the photosynthetic pigment content, compared with the control, as had been observed in Chrysanthemum morifolium shoot cultures, where such a change resulted in a decrease in the concentration of photosynthetic pigments (Kurilčik et al. 2008).
Antioxidant potential
In this study, we assessed the antioxidant potential of extracts from cultivated biomass depending on the LED light variants used in relation to white fluorescent light and darkness conditions, using three different assays—CUPRAC, DPPH, and FRAP (Table 3). The highest antioxidant potential evaluated with the CUPRAC method (2.39 mmol TE 100 g−1 DW) was demonstrated for the RBY light, and the lowest (1.65 mmol TE 100 g−1 DW) for D. In the DPPH assay, the highest antioxidant potential was recorded for WLED (0.71 mmol TE 100 g−1 DW), and the lowest for RBY (0.37 mmol TE 100 g−1 DW). In the FRAP assay, the highest antioxidant potential was obtained for the RBY light (0.40 mmol TE 100 g−1 DW) and the lowest for the R light (0.21 mmol TE 100 g−1 DW). The results showed a small influence of the applied lighting conditions on the total antioxidant potential. The antioxidant potential that had been assessed with the CUPRAC assay of extracts from N. officinale microshoot cultures grown for 20 days in RITA® bioreactors had values similar to the maximum value reached in this study (Klimek-Szczykutowicz et al. 2020a). Having analyzed these results, we can suppose that the tested lighting conditions did not significantly affect the antioxidant potential, which was probably related to their influence on the amounts of polyphenolic compounds in the N. officinale microshoot cultures.
Similar results showing little effect of different lighting conditions on the antioxidant potential of biomass extracts had also been observed in other, formerly studied, in vitro cultures. For example, Ocimum basilicum var. pupurascens callus cultures grown under different LED light spectra had been assessed for antioxidant potential with the DPPH assay (Nazir et al. 2020). No differences between light quality and antioxidant activity were shown in that assay, while in the FRAP and ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) assays the highest antioxidant activity was obtained for blue light and white light. The studies on callus cultures of Rhodiola imbricata had investigated the influence of LED light quality on antioxidant activity (Kapoor et al. 2018). Antioxidant activity was expressed by the total antioxidant capacity based on phosphomolybdenum and DPPH tests. The total antioxidant activity showed no significant differences in the antioxidant power of the biomass grown under different lighting conditions. However, the percentage inhibition of DPPH free-radical scavenging activity was higher for calluses grown under blue light, but high antioxidant potential was also confirmed for illumination with white light.
Conclusions
To sum up, this study evaluated for the first time the influence of different lighting conditions on biomass growth, the production of glucosinolates, phenolic compounds, soluble sugars, and photosynthetic pigments, as well as on antioxidant potential in agar microshoot cultures of N. officinale. The study showed a clear effect of the applied LED lighting conditions on the amounts of sulphur compounds and a slight effect on the amounts of polyphenols and the antioxidant potential of biomass extracts. The best biomass growth and high glucosinolates production was shown for microshoots grown under the combination of 50% green, 35% red, and 15% blue lights (RBG light). Considering watercress as a plant whose greatest value is the production of glucosinolates, valuable in terms of biological properties, the RBG light can be nominated as the most suitable for high-productivity N. officinale microshoot cultures. From the point of view of plant biotechnology techniques, the next steps in optimizing the growth of biomass and the production of metabolites in N. officinale in vitro cultures could be their cultivation in the RGB light in the special bioreactors, and experiments in the field of media supplementation with exogenic precursors or applied elicitation techniques.
To conclude, the RBG LED light can be considered the best light quality variant for increasing the production of secondary metabolites and the antioxidant potential of N. officinale microshoot cultures.
Abbreviations
- BA:
-
6-Benzyladenine
- CUPRAC:
-
CUPric Reducing Antioxidant Capacity
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl
- DW:
-
Dry weight
- F–C:
-
Folin–Ciocalteu
- FRAP:
-
Ferric Reducing Ability of Plasma
- FW:
-
Fresh weight
- GAL:
-
Gallic acid equivalent
- Gi:
-
Growth index
- LED:
-
Light-emitting diode
- MS:
-
Murashige and Skoog
- NAA:
-
1-Naphthaleneacetic acid
- PGRs:
-
Plant growth regulators
- PPFD:
-
Photosynthetic photon flux density
- RE:
-
Rutoside equivalent
- SIN:
-
Sinigrin equivalent
- TE:
-
Trolox equivalent
References
Afsharypuor S, Salehi M (2008) Volatile constituents of leaves and stems of Nasturtium officinale R. Br J Essent Oil Res 20:517–518. https://doi.org/10.1080/10412905.2008.9700076
Alvarenga ICA, Pacheco FV, Silva ST et al (2015) In vitro culture of Achillea millefolium L.: quality and intensity of light on growth and production of volatiles. Plant Cell Tissue Organ Cult 122:299–308. https://doi.org/10.1007/s11240-015-0766-7
Bach A, Kapczyńska A, Dziurka K, Dziurka M (2015) Phenolic compounds and carbohydrates in relation to bulb formation in Lachenalia “Ronina” and “Rupert” in vitro cultures under different lighting environments. Sci Hortic 188:23–29. https://doi.org/10.1016/j.scienta.2015.02.038
Bahramikia S, Yazdanparast R (2010) Antioxidant efficacy of Nasturtium officinale extracts using various in vitro assay systems. JAMS J Acupunct Meridian Stud 3:283–290. https://doi.org/10.1016/S2005-2901(10)60049-0
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measuer of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Biesaga-Kościelniak J, Dziurka M, Ostrowska A et al (2014) Brassinosteroid improves content of antioxidants in seeds of selected leguminous plants. Aust J Crop Sci 8:378–388
Blios MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 26:1199–1200
Boligon AA, Janovik V, Boligon AA et al (2013) HPLC analysis of polyphenolic compounds and antioxidant activity in Nasturtium officinale. Int J Food Prop 16:61–69. https://doi.org/10.1080/10942912.2010.528111
Bong SJ, Jeon J, Park YJ et al (2020) Identification and analysis of phenylpropanoid biosynthetic genes and phenylpropanoid accumulation in watercress (Nasturtium officinale R. Br.). 3 Biotech 10:1–8. https://doi.org/10.1007/s13205-020-02244-y
Casanova NA, Ariagno JI, López Nigro MM et al (2013) In vivo antigenotoxic activity of watercress juice (Nasturtium officinale) against induced DNA damage. J Appl Toxicol 33:880–885. https://doi.org/10.1002/jat.2746
Choi J, Bok G, Lee H et al (2020) Effect of red and blue LED ratio on growth and glucosinolate contents of watercress (Nasturtium officinale) in a plant factory. Hortic Sci Technol 38:474–486
da Silva RR, de Souza RR, Coimbra MC, et al (2020) Light quality on growth and phenolic compounds accumulation in Moringa oleifera L. grown in vitro. Comun Sci 11:. https://doi.org/10.14295/cs.v11i.3313
Dubois M, Gilles K, Hamilton JK et al (1951) A colorimetric method forthe determination of sugars. Nature 168:167–168
Engelen-Eigles G, Holden G, Cohen JD, Gardner G (2006) The effect of temperature, photoperiod, and light quality on gluconasturtiin concentration in watercress (Nasturtium officinale R. Br.). J Agric Food Chem 54:328–334. https://doi.org/10.1021/jf051857o
Gallaher CM, Gallaher DD, Peterson S (2012) Development and validation of a spectrophotometric method for quantification of total glucosinolates in Cruciferous vegetables. J Agric Food Chem 60:1358–1362. https://doi.org/10.1021/jf2041142
Grzegorczyk I, Wysokińska H (2008) Liquid shoot culture of Salvia officinalis L. for micropropagation and production of antioxidant compounds; effects of triacontanol. Acta Soc Bot Pol 77:99–104. https://doi.org/10.5586/asbp.2008.013
IUCN. The IUCN red list of threatened species. https://www.iucnredlist.org/. Accessed 22 Jan 2021
Jeon J, Bong SJ, Park JS et al (2017) De novo transcriptome analysis and glucosinolate profiling in watercress (Nasturtium officinale R. Br.). BMC Genomics 18:1–14. https://doi.org/10.1186/s12864-017-3792-5
Kamal KY, Khodaeiaminjan M, El-Tantawy AA et al (2020) Evaluation of growth and nutritional value of Brassica microgreens grown under red, blue and green LEDs combinations. Physiol Plant 169:625–638. https://doi.org/10.1111/ppl.13083
Kapoor S, Raghuvanshi R, Bhardwaj P et al (2018) Influence of light quality on growth, secondary metabolites production and antioxidant activity in callus culture of Rhodiola imbricata Edgew. J Photochem Photobiol B Biol 183:258–265. https://doi.org/10.1016/j.jphotobiol.2018.04.018
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plants Res 3:1222–1239
Klimek-Szczykutowicz M, Szopa A, Ekiert H (2018) Chemical composition, traditional and professional use in medicine, application in environmental protection, position in food and cosmetics industries, and biotechnological studies of Nasturtium officinale (watercress)—a review. Fitoterapia 129:283–292. https://doi.org/10.1016/j.fitote.2018.05.031
Klimek-Szczykutowicz M, Szopa A, Blicharska E et al (2019) Bioaccumulation of selected macro- and microelements and their impact on antioxidant properties and accumulation of glucosinolates and phenolic acids in in vitro cultures of Nasturtium officinale (watercress) microshoots. Food Chem 300:125184. https://doi.org/10.1016/j.foodchem.2019.125184
Klimek-Szczykutowicz M, Dziurka M, Blažević I et al (2020a) Phytochemical and biological activity studies on Nasturtium officinale (watercress) microshoot cultures grown in RITA® temporary immersion systems. Molecules 25(22):5257. https://doi.org/10.3390/molecules25225257
Klimek-Szczykutowicz M, Szopa A, Dziurka M et al (2020b) The influence of Nasturtium officinale R. Br. agar and agitated microshoot culture media on glucosinolate and phenolic acid production, and antioxidant activity. Biomolecules 10:1216. https://doi.org/10.3390/biom10091216
Kubica P, Szopa A, Prokopiuk B et al (2020) The influence of light quality on the production of bioactive metabolites—verbascoside, isoverbascoside and phenolic acids and the content of photosynthetic pigments in biomass of Verbena officinalis L. cultured in vitro. J Photochem Photobiol B Biol 203:111768. https://doi.org/10.1016/j.jphotobiol.2019.111768
Kurilčik A, Dapkūnienė S, Kurilčik G et al (2008) Effect of the photoperiod duration on the growth of Chrysanthemum plantlets in vitro. Sci Work Lith Inst Holticullture 27:39–46
Li R, Huang W, Wang X et al (2018) Effects of yellow, green, and different blue spectra on growth of potato plantlets in vitro. Hort Sci 53:541–546
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Curr Protoc Food Anal Chem 1:171–178
Mulabagal V, Tsay H (2004) Plant cell cultures—an alternative and efficient source for the production of biologically important secondary metabolites. Int J Appl Sci Eng 2:29–48
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nadeem M, Abbasi BH, Younas M et al (2019) LED-enhanced biosynthesis of biologically active ingredients in callus cultures of Ocimum basilicum. J Photochem Photobiol B Biol 190:172–178. https://doi.org/10.1016/j.jphotobiol.2018.09.011
Nazir M, Ullah MA, Younas M et al (2020) Light-mediated biosynthesis of phenylpropanoid metabolites and antioxidant potential in callus cultures of purple basil (Ocimum basilicum L. var purpurascens). Plant Cell Tissue Organ Cult 142:107–120. https://doi.org/10.1007/s11240-020-01844-z
Özyürek M, Güçlü K, Bektaşoğlu B, Apak R (2007) Spectrophotometric determination of ascorbic acid by the modified CUPRAC method with extractive separation of flavonoids–La(III) complexes. Anal Chim Acta 588:88–95. https://doi.org/10.1016/j.aca.2007.01.078
Park NI, Kim JK, Park WT et al (2011) An efficient protocol for genetic transformation of watercress (Nasturtium officinale) using Agrobacterium rhizogenes. Mol Biol Rep 38:4947–4953. https://doi.org/10.1007/s11033-010-0638-5
Ramakrishna A, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6:1720–1731. https://doi.org/10.4161/psb.6.11.17613
Ramawat KG, Mathur M (2007) Factors afecting the production of secondary metabolites. In: Ramawat KG, Merillon JM (eds) Biotechnology: secondary metabolites, plants and microbes. Science Publ Inc, Jarsey Plymouth, pp 59–102
Ramos RTM, Bezerra ICF, Ferreira MRA, Soares LAL (2017) Spectrophotometric quantification of flavonoids in herbal material, crude extract, and fractions from leaves of Eugenia uniflora Linn. Pharmacognosy Res 9:253–260. https://doi.org/10.4103/pr.pr_143_16
Sadeghi H, Mostafazadeh M, Sadeghi H et al (2014) In vivo anti-inflammatory properties of aerial parts of Nasturtium officinale. Pharm Biol 52:169–174. https://doi.org/10.3109/13880209.2013.821138
Shin KS, Murthy HN, Heo JW, Paek KY (2004) Induction of betalain pigmentation in hairy roots of red beet under different radiation sources. Biol Plant 47:149–152
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzym 299:152–178. https://doi.org/10.1016/S0076-6879(99)99017-1
Szopa A, Starzec A, Ekiert H (2018) The importance of monochromatic lights in the production of phenolic acids and flavonoids in shoot cultures of Aronia melanocarpa, Aronia arbutifolia and Aronia × prunifolia. J Photochem Photobiol B Biol 179:91–97. https://doi.org/10.1016/j.jphotobiol.2018.01.005
Ullah MA, Tungmunnithum D, Garros L et al (2019) Monochromatic lights-induced trends in antioxidant and antidiabetic polyphenol accumulation in in vitro callus cultures of Lepidium sativum L. J Photochem Photobiol B Biol 196:111505. https://doi.org/10.1016/j.jphotobiol.2019.05.002
Wainwright H, Marsh J (1986) The micropropagation of watercress (Rorippa nasturtium-aquaticum L.). J Hortic Sci 61:251–256. https://doi.org/10.1080/14620316.1986.11515698
Wielanek M, Królicka A, Bergier K et al (2009) Transformation of Nasturtium officinale, Barbarea verna and Arabis caucasica for hairy roots and glucosinolate-myrosinase system production. Biotechnol Lett 31:917–921. https://doi.org/10.1007/s10529-009-9953-0
Yehuda H, Soroka Y, Zlotkin-Frušić M et al (2012) Isothiocyanates inhibit psoriasis-related proinflammatory factors in human skin. Inflamm Res 61:735–742. https://doi.org/10.1007/s00011-012-0465-3
Zhong J, Seki T, Kinoshita S, Yoshida T (1991) Effect of light irradiation on anthocyanin production by suspended culture of Perilla frutescens. Biotechnol Bioeng 38:653–658
Funding
This study was funded by Grant Number PL: N/42/DBS/000122.
Author information
Authors and Affiliations
Contributions
MK-S, AS, BP and KD carried out the experiments, compiled the data, performed the chemical and statistical analysis. MK-S, AS, KD, BP and HE corrected and revisited the manuscript. All the authors read and approved the manuscript in its final form.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declares that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Wagner Campos Otoni.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Klimek-Szczykutowicz, M., Prokopiuk, B., Dziurka, K. et al. The influence of different wavelengths of LED light on the production of glucosinolates and phenolic compounds and the antioxidant potential in in vitro cultures of Nasturtium officinale (watercress). Plant Cell Tiss Organ Cult 149, 113–122 (2022). https://doi.org/10.1007/s11240-021-02148-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-021-02148-6