Abstract
The effectiveness of microspore embryogenesis (ME) is determined by a complex network of internal and environmental factors. In the present study on triticale and barley, strong positive correlation (r = 0.85) between the generation of hydrogen peroxide (H2O2) and ME effectiveness confirmed the important role of reactive oxygen species in microspore reprogramming. However, for high effectiveness of ME induction, intensive H2O2 generation had to be associated with high activity of antioxidative enzymes, superoxide dismutase and catalase. The strong seasonal effect on the physiological status of microspores revealed in the study suggests a kind of ‘biological clock’ controlling plant reproduction, crucial for microspore viability and embryogenic potential. Although the effect of various modifications of ME-inducing stress tiller pre-treatment was determined mainly by the physiological condition of microspores, at higher stress intensity positive effects induced by antioxidant molecules—reduced glutathione and its precursor, l-2-oxothiazolidine-4-carboxylic acid—were observed. High level of variation in the response to ME-inducing stress tiller pre-treatment was also revealed between the two DH lines of triticale and two cultivars of barley and among microspores isolated from subsequently developed spikes.
Key message
Microspore reprogramming towards embryogenic development is initiated by the genotype-specific threshold of reactive oxygen species accumulation. However, high effectiveness of the process requires efficient system of antioxidative defence.
Similar content being viewed by others
Introduction
The population of isolated and in vitro cultured microspores represents a unique, highly advantageous model for studying the mechanisms which determine the phenomenon of plant cell totipotency, dedifferentiation and reprogramming towards embryogenic development (Testillano 2019). It gives the possibility to monitor the process of embryogenesis continuously, right from the beginning, in a big population of homogeneous cells and to modify a number of environmental and endogenous factors which regulate this process. Several decades of research revealed some important facts but the precise sequence of events and all factors involved in the efficient initiation of microspore embryogenesis (ME) have not been definitely identified yet. The progress is highly anticipated as better understanding of molecular and physiological background of ME has great breeding importance (Dwivedi et al. 2015), providing the possibility for the development of doubled haploid (DH) procedures for recalcitrant genotypes and its wider incorporation into breeding programmes.
Recent interest focused on the role of redox control in plant growth and development suggests that the signal triggering microspore reprogramming is associated with the generation of reactive oxygen species (ROS). For many years, ROS were mainly considered to be undesirable by-products of aerobic metabolism able to induce serious oxidative damage of cellular components and disrupt metabolic processes, ultimately leading to cell death. However, over the last two decades the research has revealed that starting from fertilization and the first cell division, through shoot, root and flower bud formation up to senescence—all developmental phases are regulated by changes in redox status at organ, tissue, cellular and subcellular levels (Kocsy et al. 2013; Considine and Foyer 2014). Moreover, ROS play a signalling role, informing plants about stress and initiating many defence reactions (Mittler 2017; Schmitt et al. 2014, Noctor et al. 2018; Mhamdi and van Breusegem 2018). Among ROS, hydrogen peroxide (H2O2) is particularly interesting, as its ability to diffuse through cell membranes predisposes it to trigger chemical reactions and affect responsive targets, such as metabolites or proteins, inside and outside organelles of its origin. However, other ROS species such as singlet oxygen (1O2) and superoxide anion (O2·−) cannot be excluded from the group of potential candidates to play the role of signalling molecules involved in the induction of stress response mechanisms (Schmitt et al. 2014; Dietz et al. 2016; Noctor et al. 2018, Mhamdi and van Breusegem 2018).
ROS generation associated with the process of embryogenesis in in vitro system was first reported by Earnshaw and Johnson (1985, 1987), who examined the effects of antioxidants on the somatic embryo development of wild carrot. The continuation of their research resulted in the conclusion that a reduced environment stimulates cells divisions, whereas a more oxidized environment is necessary for embryo formation and patterning (reviewed in Yeung et al. 2005; Stasolla et al. 2004, 2008).
As previously mentioned, at first ROS generation associated with the initiation of ME was treated as a negative (cytotoxic) but inevitable side-effect of stress treatment. Such approach was in agreement with the observed upregulation of genes and the increase in the activity of antioxidative enzymes—i.e. superoxide dismutase (SOD) and/or catalase (CAT)—as well as the accumulation of low molecular weight antioxidants detected in embryogenic microspores (Vrinten et al. 1999; Maraschin et al. 2006; Muñoz-Amatriaín et al. 2006; Jacquard et al. 2009; Elhiti et al. 2012; Uváčková et al. 2012). Both the above-mentioned enzymes—SOD, which catalyse the dismutation of O2·− to H2O2, and CAT, which subsequently detoxifies H2O2 to O2 and H2O—help maintain the redox balance and play a crucial role in cell defence against oxidative stress. Many studies also confirmed that the effectiveness of ME was significantly increased through the application of exogenous ROS-scavenging molecules such as reduced glutathione (GSH), reduced ascorbate (Asc), proline, tocopherol or dimethyl tyrosine (DMT) conjugated peptide (Belmonte et al. 2003; Stasolla et al. 2004; Asif et al. 2013; Hoseini et al. 2014; Sinha and Eudes 2015; Zeng et al. 2017).
The new idea assuming a direct role of ROS in ME initiation can explain why microspore reprogramming can be induced by various stress factors (for example low or high temperature, starvation, osmotic or hormonal shock) as ROS generation is associated with any disturbance in cell metabolism (Mittler 2002). Dual, signalling and toxic effects of ROS overproduction are in agreement with the observations that higher stress intensity increases the effectiveness of ME initiation, but at the same time can reduce cell viability and green plant regeneration ability (Rodriguez-Serrano et al. 2012; Shariatpanahi et al. 2006). Our previous studies with the use of anther and isolated microspore cultures of triticale (Żur et al. 2008, 2009, 2012, 2014, 2019) revealed that various stress factors used for ME initiation enhanced ROS generation and induced genotype-specific changes in the activity of the antioxidative system, which could be associated with the embryogenic potential of the examined cultivar/DH line (Żur et al. 2008, 2009, 2014). We also postulated a dual role of GSH in isolated microspore cultures of triticale, which on the one hand provides direct antioxidative defence and on the other hand, creates a redox status regulating cell proliferation during early embryo-like structure (ELS) development (Żur et al. 2019).
In the present study, we continued to analyse the role of the antioxidative system in the process of microspore reprogramming towards the embryogenic pathway. Our usual model of study—DH lines of winter triticale—was supplemented with two cultivars of barley: cv. Igri combining high embryogenic potential with high green plant regeneration efficiency (Larsen et al. 1991) and cv. Golden Promise, recalcitrant to ME under conventional low temperature treatment (Coronado et al. 2005; Lippmann et al. 2015).
The primary objective of our research was to identify common and specific physiological phenomena associated with ME induction in relation to genetic (different crop species) and environmental (various ME-inducing treatments) variation. The effect of standardly used low temperature tiller pre-treatment (3 weeks at 4 °C; LT) was modified with: (1) GSH, (2) GSH precursor (l-2-oxothiazolidine-4-carboxylic acid, OTC) and (3) buthionine sulfoximine (BSO)—irreversible inhibitor of γ-glutamylcysteine synthetase, the rate-limiting enzyme for GSH de novo synthesis. We also applied two inhibitors of antioxidative enzymes: diethyldithiocarbamate trihydrate (DDC)—Cu2+ chelating agent which inhibits SOD activity, and 3-amino-1,2,4-triazole (3-AT) which inhibits CAT activity binding covalently to the heme-containing active centre. All of the used chemicals were analysed with respect to microspore viability, the effectiveness of ME induction and cellular redox status regulated by the balance between ROS generation and antioxidative defence.
Material and methods
Plant material and growth conditions
The research was done into two DH lines of winter triticale (× Triticosecale Wittm.) derived from the F1 generation of a cross between German inbred line Saka 3006 and Polish cv. Modus (responsive DH28 and recalcitrant DH19) and two barley (Hordeum vulgare L.) cultivars (responsive winter cv. Igri and recalcitrant spring cv. Golden Promise). The seeds of DH lines of triticale were obtained from the State Breeding Institute at the University of Hohenheim (Germany), whereas the seeds of barley cultivars were obtained from Leibniz Institute of Plant Genetics and Crop Plant Research (Gatersleben, Germany).
Kernels of triticale and winter barley cv. Igri were germinated for 2 days at 20 °C in perlite pre-soaked with Hoagland’s salt solution (HS; according to Wędzony 2003) and vernalized for 7 weeks at 4 °C and 8 h/16 h (day/night) photoperiod. Kernels of spring cv. Golden Promise were germinated under the same conditions and kept at 4 °C for 2 weeks. Cold-treated seedlings were planted into pots containing a mixture of soil and sand (3/1; v/v), and grown in a glasshouse at 20 °C and 16 h/8 h photoperiod. Additional illumination with the irradiance of 400 μmol m−2 s−1 was supplied by high pressure sodium (HPS) lamps SON-T + AGRO (Philips, Brussels, Belgium) during unfavorable growth conditions.
A series of four experiments were carried out over the course of two years. The experiments were conducted over different growing seasons, with microspore isolation starting in May/July (Exp. 1), November (Exp. 2), April (in the case of cv. Igri prolonged to June; Exp. 3), and March (Exp. 4).
Tiller pre-treatment
Tillers were harvested at the phase when the majority of microspores were at mid- to late uni-nucleate stage of development, placed in HS medium and stored for 3 weeks at 4 °C in the dark. In Exps. 1 and 3, 4 days before microspore isolation, a part of tillers was transferred to fresh HS medium supplemented with 0.3 mmol dm−3 GSH (LT + GSH), 0.3 mol dm−3 mannitol (LT + MAN) or both GSH and mannitol (LT + MAN + GSH). The control tillers were transferred to fresh HS medium (LT).
In Exp. 2, the scheme of the tiller pre-treatment was similar, but HS medium was supplemented with: (1) diethyldithiocarbamate trihydrate (Cat. No. 228680, Sigma-Aldrich) (LT + DDC); (2) 3-amino-1,2,4-triazole (Cat. No. A8056, Sigma-Aldrich) (LT + 3-AT), (3) buthionine sulfoximine (Cat. No. B2515, Sigma-Aldrich) (LT + BSO), and (4) l-2-oxothiazolidine-4-carboxylic acid (Cat. No. O6254, Sigma-Aldrich) (LT + OTC). All the chemicals were applied at 0.25 mmol dm−3 concentration selected based on the literature data. The control tillers were transferred to fresh HS medium (LT).
In Exp. 4, the effects of two substances which produced the most interesting results in Exp. 3—OTC and DDC—were analysed again with the use of the same experimental scheme.
Microspore isolation and culture
Depending on plant growing season and plant species, 20–40 and 20–80 spikes were used for one isolation procedure for triticale and barley, respectively.
The procedure of triticale microspore isolation was described in detail in Żur et al. (2019). The isolation of barley microspores was performed according to the same method with the exception of the concentration of MAN, which was increased to 0.4 mol dm−3. The total number of collected microspores was estimated using a Neubauer counting chamber, then the suspension was sampled for in vitro culture and biochemical analysis. Induction media 190-2 (Zhuang and Xu 1983) modified according to Pauk et al. (2003) and KBP (Kumlehn et al. 2006) were used for isolated microspore cultures of triticale and barley, respectively.
Microspore suspensions with the final density of approximately 7 × 104 microspores per ml (mcs ml−1) for triticale and 105 mcs ml−1 for barley were transferred to 15 × 60 mm Petri dishes. Microspores of triticale were co-cultured with isolated ovaries dissected simultaneously with microspores (10 per 1 ml of suspension). All cultures were incubated in the dark at 26 °C.
Starting from the sixth week of culture, ELS of triticale over 1 mm in size were transferred to 0.6% agar solidified regeneration medium 190-2 (Zhuang and Xu 1983) supplemented with 0.5 mg dm−3 kinetin, 0.5 mg dm−3 NAA and 30 g dm−3 sucrose, pH 6.0.
ELS of barley were transferred onto KBP4P medium for two weeks, then to K4NBT according to the procedure described by Otto et al. (2015). All cultures were incubated at 26 °C, with 16 h/8 h (day/night) photoperiod in dim light—at about 30 μmol (hν) m−2 s−1 (PAR) during the first week, then transferred to 80–100 μmol (hν) m−2 s−1 (PAR).
Measurements of cytological parameters associated with microspore embryogenesis initiation and further stages of embryogenic development
The number of isolated microspores received per one spike of the donor plant was considered as ‘the microspore yield’.
Microspore viability was determined through fluorochromatic reaction to fluorescein diacetate (FDA; 0.01%; λEx = 465 nm, λEm = 515 nm, green fluorescence; Heslop-Harrison and Heslop-Harrison 1970). The samples were examined with Nikon Eclipse E600 epifluorescence microscope equipped with Zyla 4.2 (Andor) camera. Photos were taken using NIS-Elements AR 4.00 software. At least 500 microspores from 10 fields of view (magnification ×10) for each individual sample were evaluated. Microspore samples were standardly collected at the end of the isolation procedure. Only in Exp. 2, the samples were collected twice: (1) after the first centrifugation from crude sediment of homogenised spikes, and (2) at the end of the isolation, after the washing procedure separating somatic tissue debris and dead or damaged microspores from viable cells.
Progressive stages of ME were monitored at five time points—on the isolation day (0 day) and on the 8th, 14th, 28th and 42th day of in vitro culture. Observations were made at least for 10 fields of view per one Petri dish using Nikon Eclipse TS100 inverted microscope and processed by DS-Ri1 digital camera and NIS-Elements AR 4.0 software. The number of ELS over 1 mm in size was assessed after 6 weeks of in vitro culture and calculated per one spike of the donor plant (ELS per spike).
Measurements of antioxidative enzymes activities
The activities of antioxidative enzymes were determined in freshly isolated microspores after 1 h incubation at 26 °C in the dark. The samples of microspores suspended in the medium were centrifuged for 3 min at 500 rpm at room temperature. Collected pellet was homogenized by vortexing (90 s) with glass grinding balls in 50 mM potassium phosphate buffer (PPB, pH 7.5), supplemented with 1 mM EDTA, in the proportion of 3 ml buffer for 1 g microspores. The homogenate was centrifuged at 12,000 rpm for 3 min. Protein content was determined in the supernatant according to Bradford (1976) using bovine serum albumin (BSA) as the standard.
The activity of superoxide dismutase (SOD, EC 1.15.11) was registered at λ = 550 nm using the modified method of McCord and Fridovich (1969), where one unit of SOD activity was expressed as the amount of the enzyme required to cause 50% inhibition of cytochrome c in a coupled system, using xanthine and xanthine oxidase at pH 7.8 and 25 °C in a 1.0 ml reaction volume. Xanthine oxidase was used in the concentration which produced an initial (uninhibited) ΔA 550 nm of 0.025 ± 0.005 min−1.
The activity of catalase (CAT, EC 1.11.1.6) was determined at λ = 240 nm according to Aebi (1984). The reaction mixture contained 50 mM PPB buffer (pH 7.0), 0.1 mM EDTA, 0.04% (v/v) H2O2 and 35 μl enzyme extract in 0.7 ml total reaction volume. A unit of enzyme activity (U) was defined as a decrease in absorbance at 25 °C equal to 0.0145 (consumption of 1 μM of H2O2).
All measurements were carried out using a ULTROSPEC 2100 PRO (GE Healthcare Life Sciences) UV-Vis spectrophotometer in at least three biological replicates.
Determination of endogenous H2O2 content
The concentration of H2O2 in microspores was measured using the Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit by Invitrogen (Waltham, Massachusetts, USA).
After isolation, triticale and barley microspores were incubated in the induction media for 1 h at 26 °C in the dark. Then, microspores were washed with 50 mM Tris HCl with 1 mM EDTA buffer (pH 7.4) and homogenised in the ice-cold phosphate buffered saline (PBS, provided in the kit) at the ratio 1:5 (w/v). The obtained homogenates were centrifuged (10,000 × g, 4 min at 4 °C) and H2O2 concentration was measured colorimetrically in the supernatant according to the manufacturer’s protocol using a microplate reader (Synergy 2; BioTek, Winooski, Vermont, USA) under λEx = 530 nm and λEm = 590 nm. The standard curve in the range 0.05–1 μg dm−3 was obtained using different concentrations of H2O2 provided in the kit.
Statistical analysis
The effect of normally distributed variables was examined by one-factor analysis of variance (ANOVA), after which post-hoc comparison was conducted using Duncan’s multiple range test (p ≤ 0.05). Data expressed as percentages were first transformed using arcsine transformation. Pearson correlation coefficients (r) were used to analyze associations between variables. All statistical analyses were performed using STATISTICA version 10.0 package (StatSoft, USA, 2011).
Results
The influence of genotype and environmental factors on the microspore yield, viability and the effectiveness of ME induced by low-temperature (control) tiller pre-treatment
Significant variation among the studied plant materials with respect to their embryogenic potential was fully confirmed in the conducted experiments. Several parameters given below distinctly differentiated the isolated microspores—both common and distinct for the examined plant species.
The first visible difference among the studied plant materials was the yield of isolated microspores received after the standardly used LT tiller pre-treatment (21 days at 4 °C, control), calculated as the mean number of microspores isolated per individual spike. Comparing the two studied cereal species, triticale was characterized by a significantly higher microspore yield (Tables 1 and 2) with the mean number of ca. 111,000 microspores per individual spike in comparison with 18,000 characteristic for barley. No significant difference was detected between DH lines of triticale, while 1.3–2.6–fold higher number of microspores was received from a spike of cv. Igri in comparison with cv. Golden Promise (Tables 1 and 2).
The viability of cells measured after LT treatment in crude microspore suspensions (estimated in Exp. 2) varied significantly from 24% to 37% (Supplementary Source 1) but was not associated with embryogenic potential. The washing procedure based on density gradient centrifugation resulted in a mean two-fold increase in the number of viable cells in comparison to crude microspore suspensions. Moreover, after the washing procedure a significantly higher microspore viability was detected in the responsive line DH28 and cv. Igri in comparison with the recalcitrant DH19 and cv. Golden Promise.
For the majority of the studied genotypes, the frequency of viable microspores in suspensions isolated from LT pre-treated tillers depended significantly on the donor plant’s growing season (Fig. 1). Both winter triticale DH lines turned out to be highly sensitive to the environmental factors. Mean microspore viability decreased significantly from 52% to 69% received in the experiments when isolation procedures started in May/July (Exp. 1) or November (Exp. 2), through 38–42% detected in April, to 15–22% received in March (Exp. 4). In contrast, mean viability of microspores of spring barley (cv. Golden Promise), which varied from 33% to 69%, was the highest in March (Exp. 4) and the lowest in May/July (Exp. 1). Seasonal variation was not detected in microspore viability of cv. Igri, which changed to a minor extent—from 50% to 68%. In addition, variation in microspore viability was found even within each experiment between subsequent isolation procedures. Depending on the growing season and the donor plant’s genotype, the highest number of viable cells was detected in microspore population isolated from the primary tillers, secondary tillers or from tillers developed from next axillary buds (Fig. 1). Characteristic for cv. Golden Promise, in the majority of experiments, was a time-sensitive decrease in microspore viability. To eliminate the variation originating from the effect of tiller order, microspore viability and the yield of isolated microspores were also presented as the percentage of the control (Tables 1 and 2; Figs. 2 and 4).
Seasonal effect on microspore viability and its fluctuations in subsequent isolations from the primary tillers (1), secondary tillers (2) and tillers developed from next axillary buds (3). Mean viability represents means from three biological replications (isolations) ± SE. Data for individual isolations are presented in colour according to an arbitrary adopted scale (presented below). Statistical analysis was performed separately for each DH line/cultivar. Data marked with different letters differ significantly from the control according to the Duncan test (p ≤ 0.05); NS—variation not significant; n.a.—not analysed. Experiment 1 with the isolation of microspores started in May/July; Experiment 2 with the isolation of microspores started in November; Experiment 3 with the isolation of microspores started in April/June; Experiment 4 with the isolation of microspores started in March
Microspore viability of two DH lines of winter triticale (responsive DH28 and recalcitrant DH19) and two cultivars of barley (responsive winter cv. Igri and recalcitrant spring cv. Golden Promise) after low temperature (LT) tillers pre-treatment (control) and LT treatment combined with the application reduced glutathione (GSH) and/or mannitol (MAN). Microspores stained with FDA (fluorescence of vital cells intensely green). Bar = 50 μm. Included are normalized values of microspore viability. Results received in Experiment 1 with the isolation of microspores started in May/July. LT—low-temperature tiller pre-treatment (21 days at 4 °C); LT + GSH—low-temperature tiller pre-treatment combined with the application of 0.3 mmol dm−3 reduced glutathione; LT + MAN—low-temperature tiller pre-treatment combined with the application of 0.3 mol dm−3 mannitol; LT + MAN + GSH—low-temperature tiller pre-treatment combined with the application of 0.3 mol dm−3 mannitol and 0.3 mmol dm−3 reduced glutathione. All chemicals were applied during the last 4 days before microspore isolation
Significant variation was also observed in the response of the studied cultivars/DH lines to the standard ME-inducing treatments (control, Tables 1 and 2). In Exp. 1, where the isolation of microspores started in May/July, the effectiveness of ME induction was highest for cv. Igri (>300 ELS per spike), moderate for DH28 (ca. 30 ELS per spike) and very low for both recalcitrant genotypes: DH19 and cv. Golden Promise (<1 ELS per spike). However, ME effectiveness was significantly influenced by the donor plant’s growing season and varied significantly among the conducted experiments (Tables 1 and 2). This effect was the most pronounced in the case of cv. Igri as its huge embryogenic potential observed when the isolation of microspores started in May/July was not revealed in March/April and November (Exps. 2, 3 and 4). It was clearly visible in Exp. 3 where the cultivation of cv. Igri was prolonged to June, and the effectiveness of ME induction varied drastically from about 1 to over 600 ELS per spike in April and June, respectively (Table 1). Similarly in the responsive triticale line DH28, the effectiveness of ME initiation varied in all conducted experiments from 0 to 144 ELS per spike (Tables 1 and 2).
The influence of stress on the microspore yield, viability and the effectiveness of ME induction (Exps. 1 and 3)
The effects induced by various stress treatments were genotype-specific. Generally, modifications introduced in Exp. 1 had no effect on the microspore yield (Table 1). The only exception was LT + MAN treatment applied to tillers of cv. Igri, which increased the number of isolated microspores, but at the same time significantly decreased their viability (Figs. 1 and 2) and dramatically diminished the number of produced ELS (numerical data in Table 1; exemplary pictures in Fig. 3). No other variation was observed in both responsive genotypes (cv. Igri, DH28) with respect to microspore viability. In both recalcitrant genotypes, microspore viability was enhanced when LT was combined with the application of exogenous GSH, alone or concomitantly with MAN (Fig. 2). Only in DH19, also LT + MAN significantly increased the frequency of viable microspores.
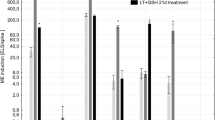
The effect of low temperature (LT, control) tillers pre-treatment and LT treatment combined with the application of reduced glutathione (GSH) and/or mannitol (MAN) on the effectiveness of ME in in vitro cultures of two DH lines of winter triticale (responsive DH28 and recalcitrant DH19) and two cultivars of barley (responsive winter cv. Igri and recalcitrant spring cv. Golden Promise). Results received in Experiment 1 with the isolation of microspores started in May/July. LT—low-temperature tiller pre-treatment (21 days at 4 °C); LT + GSH—low-temperature tiller pre-treatment combined with the application of 0.3 mmol dm−3 reduced glutathione; LT + MAN—low-temperature tiller pre-treatment combined with the application of 0.3 mol dm−3 mannitol; LT + MAN + GSH—low-temperature tiller pre-treatment combined with the application of 0.3 mol dm−3 mannitol and 0.3 mmol dm−3 reduced glutathione. All chemicals were applied during the last 4 days before microspore isolation
None of the treatments effectively induced the process of ME in isolated microspore cultures of highly recalcitrant DH19 and cv. Golden Promise (Table 1). At the same time, the only one significant effect in microspore cultures of DH28 was induced by exogenously applied GSH resulting in almost four times higher production of ELS. This effect was not observed in isolated microspore cultures of cv. Igri. However in this case, application of GSH concomitantly with MAN reversed the dramatic decrease in the effectiveness of ELS production observed after MAN treatment (Table 1, Fig. 3).
Interestingly, the effects of the same treatments applied in the second replication of the experiment (Exp. 3) were not completely reproducible (Table 1). The received results confirmed strong genotype control of the microspore yield, not influenced by any of the applied modifications. Again, this parameter was significantly higher in triticale in comparison with both barley cultivars (Table 1). However, positive effects of LT + GSH and LT + GS + MAN on microspore viability of the recalcitrant genotypes (DH19 and cv. Golden Promise) were not observed (Fig. 1). On the contrary, these treatments significantly diminished (by about 20% in comparison with the control) the number of viable cells in DH19 while only LT + MAN + GSH enhanced significantly (by about 70%) cell viability of cv. Golden Promise. Surprisingly, this had no effect on the effectiveness of ME induction. The strong negative effect of LT + MAN on microspore viability of cv. Igri and on the effectiveness of ME initiation observed in Exp. 1 was not confirmed either (Table 1, Fig. 1). This time, the negative effect of LT + GSH and LT + MAN application on ELS production was observed in microspore cultures of DH28 and similarly it was, at least partially, reversed by combined tiller pre-treatment (LT + MAN + GSH; Table 1).
The influence of disturbances in antioxidative defense system on the microspore yield, viability and the effectiveness of ME induction (Exps. 2 and 4)
The effects of two inhibitors of antioxidative enzymes (DDC, 3-AT) and two modulators of endogenous glutathione biosynthesis (BSO, OTC) were examined in Exp. 2, with microspore isolation procedures starting in November. Once again, the applied modifications did not induce significant changes in the microspore yield (Table 2). The only exception was the treatment with BSO, which increased by around 70% the number of isolated microspores per spike of the responsive DH28. Interestingly, although at the same time BSO diminished the number of viable cells estimated after the washing procedure (numerical data in Fig. 1; exemplary pictures in Fig. 4), it did not reduce the effectiveness of ELS formation (Table 2). Generally, the viability of microspores after LT tiller pre-treatment was relatively high, ranging from 55% to 69% of total microspore populations (Fig. 1). All tested chemicals induced genotype-specific and usually negative effects on microspore viability (Figs. 1 and 4). The exception was cv. Igri, for which tiller pre-treatments with BSO, OTC and 3-AT significantly increased the number of viable cells by about 18-33% in comparison with the control (LT).
Microspore viability of two DH lines of winter triticale (responsive DH28 and recalcitrant DH19) and two cultivars of barley (responsive winter cv. Igri and recalcitrant spring cv. Golden Promise) after LT tillers pre-treatment (control) and LT treatment combined with the application of chemicals inducing disturbances in the activity of the antioxidative system. Results received in Experiment 2 with the isolation of microspores started in November. Microspores stained with FDA (fluorescence of vital cells intensely green). Bar = 50 μm. Included are normalized values of microspore viability. LT + DDC—low temperature tillers pre-treatment combined with the application of 0.25 mmol dm−3 diethyldithiocarbamate trihydrate; LT + 3AT—low temperature tillers pre-treatment combined with the application of 0.25 mmol dm−3 3-amino-1,2,4-triazole; LT + OTC—low temperature tillers pre-treatment combined with the application of 0.25 mmol dm−3 l-2-oxothiazolidine-4-carboxylic acid; LT + BSO—low temperature tillers pre-treatment combined with the application of 0.25 mmol dm−3 buthionine sulfoximine. All chemicals were applied during the last 4 days before microspore isolation
The effectiveness of ME induction acquired in Exp. 2 is presented in numerical form in Table 2 and exemplary pictures are shown in Fig. 5. For control (LT) tiller pre-treatment, the number of produced ELSs was similar (for cv. Golden Promise) or even higher (for DH28 and DH19) when compared to earlier results in Exp. 1. At the same time the standardly high embryogenic potential of cv. Igri was not expressed. Among various tested chemicals, DDC had no significant effect on triticale ELS formation, although some positive effects seem to be induced in the case of DH19 (Table 2). The same treatment completely inhibited ELS production in both barley cultivars. Conversely, treatment with OTC visibly diminished embryogenic potential of DH28 but increased ELS production in isolated microspore cultures of cv. Golden Promise. The treatments with 3-AT and BSO had no significant effect on ELS production.
The effect of low temperature (LT, control) tillers pre-treatment and LT treatment combined with the application of chemicals inducing disturbances in the activity of antioxidative system on the effectiveness of ELS formation in two DH lines of winter triticale (responsive DH28 and recalcitrant DH19) and two cultivars of barley (responsive winter cv. Igri and recalcitrant spring cv. Golden Promise). Results received in Experiment 2 with the isolation of microspores started in November. LT + DDC—low temperature tillers pre-treatment combined with the application of 0.25 mmol dm−3 diethyldithiocarbamate trihydrate; LT + 3AT—low temperature tillers pre-treatment combined with the application of 0.25 mmol dm−3 3-amino-1,2,4-triazole; LT + OTC—low temperature tillers pre-treatment combined with the application of 0.25 mmol dm−3 l-2-oxothiazolidine-4-carboxylic acid; LT + BSO—low temperature tillers pre-treatment combined with the application of 0.25 mmol dm−3 buthionine sulfoximine. All chemicals were applied during the last 4 days before microspore isolation
Two treatments (LT + DDC, LT + OTC) inducing diverse effects on triticale/barley ME initiation were used again in Exp. 4. Here, the isolation of microspores started in March, viability assay revealed only 15–22% of viable triticale microspores after LT tiller pre-treatment (Fig. 1). At the same time, viability of barley microspores ranged from 54% to 69%. Mean microspore viability for both species varied from 42% observed in control tillers to 31% in DDC- and 52% in OTC-treated tillers. For both studied DH lines of triticale, OTC significantly increased the number of viable cells by 21–48% in comparison with the control, which however, had no effect on ME effectiveness (Table 2). Moreover, although the viability of microspores of both barley cultivars was relatively high, again the usually high embryogenic potential of cv. Igri was not expressed.
Seasonal and stress-induced variation in the activity of antioxidative enzymes and hydrogen peroxide generation
The generation of H2O2 and the activity of two major antioxidative enzymes—SOD and CAT—were assayed twice, in Exps. 3 and 4.
LT (control) tiller pre-treatment applied in Exp. 3 resulted in relatively high activity of SOD detected in the microspores of responsive genotypes: cv. Igri and DH28 (62–78 U mg−1 protein) in comparison with recalcitrant ones: DH19 and cv. Golden Promise (18–36 U mg−1 protein; Fig. 6a). Moreover, a significant variation associated with embryogenic potential was observed between the studied triticale genotypes, as more than four times higher activity of this enzyme was revealed in microspores of the responsive DH28 in comparison with the recalcitrant DH19. A similar trend was observed in barley, but higher variation made this effect statistically insignificant. At the same time, all studied DH lines/cultivars were characterized by a relatively similar CAT activity (60–80 μmol H2O2 min−1 mg−1 protein; Fig. 6b). In the case of triticale, also the level of H2O2 generated in response to LT was significantly different and drastically higher in microspores of the responsive DH28 than in DH19 (Fig. 6c). In contrast, a significantly lower level of H2O2 was detected in microspores of the responsive barley cv. Igri compared to cv. Golden Promise, although the variation range was much smaller.
Activity of SOD (a, d) and CAT (b, e), and H2O2 generation (c, f) in microspores of two DH lines of winter triticale (responsive DH28 and recalcitrant DH19) and two cultivars of barley (responsive winter cv. Igri and recalcitrant spring cv. Golden Promise) isolated after various tillers pre-treatments. Data represent means from three biological replications (isolations) ± SE. Data marked with the same letter do not differ according to Duncan’s multiple range test (p ≤ 0.05). Figures a–c represent data received in Experiment 3 with the isolation of microspores started in April/June, figures d–f—data received in Experiment 4 with the isolation of microspores started in March. LT—low temperature tillers pre-treatment (21 days at 4 °C); LT + GSH—low temperature tillers pre-treatment combined with the application of 0.3 mmol dm−3 reduced glutathione; LT + MAN—low temperature tillers pre-treatment combined with the application of 0.3 mol dm−3 mannitol; LT + MAN + GSH—low temperature tillers pre-treatment combined with the application of 0.3 mol dm−3 mannitol and 0.3 mmol dm−3 reduced glutathione; LT + DDC—low temperature tillers pre-treatment combined with the application of 0.25 mmol dm−3 diethyldithiocarbamate trihydrate; LT + OTC—low temperature tillers pre-treatment combined with the application of 0.25 mmol dm−3 l-2-oxothiazolidine-4-carboxylic acid. All chemicals were applied during the last 4 days before microspore isolation
The effects induced by the applied modifications were genotype-specific (Fig. 6a–c). In DH28, the effect induced by LT + MAN + GSH treatment was significant, resulting in the highest SOD activity (110 U mg−1 protein) and a moderate level of H2O2 generation (15 nmol g−1 FW; Fig. 6a, c). Although other modifications of the procedure had no significant effect on antioxidative enzymes activities, the level of H2O2 generation in DH28 microspores was significantly reduced, especially in microspores isolated from LT + GSH pre-treated tillers (Fig. 6c).
No significant difference in CAT activity was observed in comparison with the control (LT) in any studied DH line/cultivar (Fig. 6b).
Regardless of the treatment, consistently low SOD activity was characteristic for isolated microspores of DH19 (18–23 U mg−1 protein; Fig. 6a). It was associated with relatively low level of H2O2 generation, especially in microspores isolated from control tillers (0.1 nmol H2O2 g−1 FW; Fig. 6c), whereas the amount of H2O2 generated in microspores isolated from LT + MAN pre-treated tillers was significantly higher (ca. 10 nmol H2O2 g−1 FW).
Two of the applied treatments—LT + MAN and LT + MAN + GSH—significantly increased SOD activity in microspores of cv. Igri in comparison with the control (LT, Fig. 6a). The effect of LT + MAN was most pronounced, resulting in the highest activity of both antioxidative enzymes and the highest level of H2O2 generation (Fig. 6a–c). Interestingly, although combined LT + MAN + GSH treatment also activated SOD, the amount of generated H2O2 diminished significantly.
All modifications reduced SOD activity in microspores of cv. Golden Promise (Fig. 6a). However, this effect did not correlate with H2O2 generation as both higher (by 48% under LT + MAN + GSH treatment) and lower (by 87% under LT + GSH treatment) amount of H2O2 was detected in comparison with the control (Fig. 6a, c).
Analyses conducted in Exp. 4 revealed that microspores of the responsive genotypes (DH28, cv. Igri) demonstrated significantly lower activity of antioxidative enzymes in comparison with the previous experiment (Fig. 6a, b, d, e). Interestingly, this time, a much higher activity of SOD was detected in microspores of the recalcitrant cv. Golden Promise, which, was not associated with a higher activity of CAT (Fig. 6c, d). In both experiments, microspores of DH19 were characterized by a low SOD activity (Fig. 6a, c), whereas a relatively high CAT activity detected in Exp. 3 was not observed this time (Fig. 6b, d). Comparing both experiments it could be noticed that LT tiller pre-treatment induced lower (by 60%) accumulation of H2O2 in microspores of DH28, but drastically increased its generation in microspores of DH19 (from 0.1 to 53 nmol g−1 FW) and cv. Golden Promise (from 15 to 134 nmol g−1 FW). Significant variation between the studied genotypes was observed only in barley, again with higher H2O2 accumulation detected in microspores of cv. Golden Promise.
The effect of tiller pre-treatment modifications applied in Exp. 4 was significant only in antioxidative defense of cv. Golden Promise (Fig. 6d, e). Interestingly, both the decrease in SOD activity induced by LT + OTC treatment and the decrease in CAT activity resulting from LT + DDC treatment had no effect on H2O2 generation (Fig. 6f).
Correlation analysis
Data received in Exp. 3 revealed a significant positive correlation between H2O2 generation and ME effectiveness (r = 0.85, p ≤ 0.05; Table 3). Moreover, a negative correlation between the microspore yield and cell viability was also detected (r = − 0.75).
In Exp. 4, the microspore yield was not only strongly negatively correlated with microspore viability (r = − 0.92) but also with the effectiveness of ME induction (r = −0 .61). Negative correlation was also found between SOD activity and the microspore yield (r = − 0.74). At the same time, the activity of this enzyme was positively correlated with the viability of microspores (r = 0.60) and H2O2 generation (r = 0.81). Conversely, CAT activity was positively correlated with the microspore yield (r = 0.78) but negatively with its viability (r = −0.75) and ME effectiveness (r = − 0.79).
Discussion
Despite years of research, many aspects of ME regulation on molecular, cytological and physiological levels have yet to be fully explained. It remains unclear why not only various plant species or cultivars, but even closely related plant genotypes differ significantly with respect to their embryogenic potential. Such variation was observed, for example in the triticale mapping population composed of 90 DH lines derived from the cross Saka 3006 × Modus, being the standard model of our studies (Krzewska et al. 2012, 2015). Moreover, this kind of variation was also revealed among individual plants of the same genotype and among subsequent spikes produced by an individual plant. Several authors also reported strong seasonal variation in ME effectiveness (Wang et al. 2000; Ritala et al. 2001; Datta 2005; Jacquard et al. 2006; Ercan et al. 2006; Silva 2010). The answers to these questions would be very interesting but they are not easy to obtain due to the multifactorial nature of the process and the complex network of interactions between genetic and environmental factors controlling and determining its effectiveness. The data presented in this work can help understand why even subtle changes in the physiological condition of the donor plant associated with a disturbed redox balance can have a huge impact on the effectiveness of microspore reprogramming towards embryogenic development.
The two sibling DH lines of winter triticale (DH19, DH28) used in the study were selected as significantly different in ME induction effectiveness based on the results of our previous research (Krzewska et al. 2012, 2015; Żur et al. 2014, 2015). The two cultivars of barley (spring cv. Golden Promise and winter cv. Igri) were selected according to the same criterion based on literature data (Coronado et al. 2005; Lippmann et al. 2015). Our study confirmed significant differences between the studied plant species as well as between individual DH lines/cultivars with respect to several parameters determining ME effectiveness. We also observed a substantial effect of the donor plant’s growing season, tiller order and stress tiller pre-treatment on microspore viability, effectiveness of microspore reprogramming and the activity of the antioxidative system.
The first tested parameter—the microspore yield, dependent on microspore productivity and their condition—was determined mainly genetically and remained almost unchanged across different growing seasons and tiller pre-treatments. Although both studied plant species are predominantly self-pollinating, higher microspore productivity in triticale is probably inherited from rye, its cross-pollinated parent (Nguyen et al. 2015). Rye anthers are relatively long and produce around 19,000 pollen grains per anther, which is roughly ten times higher in comparison with wheat pollen production. Surprisingly, this parameter was not only uncorrelated with ME effectiveness but also negatively correlated with microspore viability measured through microspore enzymatic activity and cell membrane integrity (FDA assay). This result, obtained also in our earlier study (Żur et al. 2019), suggests that the washing procedure based on cell density gradient centrifugation is not so effective in the separation of fully viable microspores. On the other hand, a very low microspore yield revealed for cv. Golden Promise associated with middling or low cell viability makes it difficult to establish isolated microspore cultures. It is also important to note that only for this cultivar the quality of the produced microspores declined visibly with the tillers developed from the next axillary buds. A similar observation on barley was described by Wang et al. (2000) and Jacquard et al. (2006), who recommended the first three-five spikes as the source of donor plant material. Observations made on winter wheat showed that the timing of tiller initiation affected leaf development and influenced tiller size, the number of kernels per spike and their contribution to the final yield (Tilley et al. 2019).
For the majority of the studied DH lines/cultivars (with the exception of cv. Igri), the viability of microspores was strongly influenced by the donor plant’s growing season. Equally high viability of winter triticale microspores was received when microspore isolation started in May/July and November, with strong reduction in cell viability observed in March. In contrast, microspore viability of spring cv. Golden Promise declined from March through November to July. It should be underlined that decreased microspore viability did not result from weakened physiological condition of donor plants as no morphological symptoms of any (a)biotic stress were observed.
This seasonal effect was observed not only with respect to microspore viability but also their embryogenic potential. The most effective ME induction took place in November for both triticale DH lines and spring barley or in June in the case of winter barley. The observed phenomenon is probably determined by a kind of biological clock regulating the natural rhythm of plant development. This type of dependency has often been reported by authors using in vitro cultures as models for their study (e.g. Ritala et al. 2001; Jacquard et al. 2006; Ercan et al. 2006; Silva 2010; Ari et al. 2016). In this case, with plants growing in glass chambers illuminated additionally by HPS lamps SON-T + AGRO (Philips), the signalling system may be at least partially, based on light as its integration with other environmental stimuli determines plant adaptation to the changing environment. Using several photoreceptors (phytochromes, cryptochromes, phototropins, UV-A and UV-B detecting receptors), plants can not only measure the quantity of light but also react to subtle changes in its quality, direction and periodicity (Franklin et al. 2014; Fernando and Schroeder 2016). All these parameters changed significantly between different growth seasons, as did the ratio between the quantity of natural and artificial light. Published data confirmed that light intensity and spectrum regulated photosynthetic activity, accumulation of amino acids and antioxidants (phenolic compounds, ascorbic acid and glutathione) what significantly influenced on plant growth and development (Lee et al. 2010; Toldi et al. 2019).
Regardless of the source and perception of the environmental stimulus, its transduction regulating plant cell fate and the direction of its development is based on ROS generation and the functioning of the antioxidative system. Our earlier reports suggested such direct involvement of ROS in the process of microspore reprogramming and ME initiation (Żur et al. 2009, 2014, 2019; Zieliński et al. 2020) and this study provided new data confirming this assumption.
Threats connected with excessive ROS generation and oxidative stress initiation were discovered many years ago (Mittler 2002 and references therein). In contrast, the majority of recently published reports focus on the positive role of ROS in the regulation of cellular metabolism and initiation of defence mechanisms in response to environmental stimuli (reviewed in Kocsy et al. 2013; Mittler 2017). A sustainable level of ROS creates balanced redox environment, which enables proper functioning of numerous processes essential for life. Moreover, ROS generation is integrated with plant growth regulators (PGRs)—auxins, cytokinins, gibberellins, abscisic acid, ethylene, brassinosteroids, strigolactones, salicylic acid and jasmonic acid—in the coordination of plant growth, development and stress adaptation (Xia et al. 2015; Arnaund et al. 2017). It was observed that too low level of ROS could suppress cell proliferation, differentiation and diminished the level of stress tolerance. However, for many years, ROS accumulation was examined only as an undesirable by-product of stress used for the induction of ME. Based on the results of our earlier studies, we postulated that one of the most important prerequisites for effective ME induction is the ability to sustain oxidative stress generated by ROS accumulation associated with the procedure of tiller pre-treatment, microspore isolation and transfer to in vitro culture conditions (Żur et al. 2009, 2014, 2019; Zieliński et al. 2020). Such stress tolerance should be connected with a relatively high activity of the antioxidative system. However, it was also underlined that a certain threshold level of ROS seems to be necessary for microspore reprogramming and effective ME initiation (Żur et al. 2014). To verify this hypothesis, this study analysed the effects of various stress tiller pre-treatments on the effectiveness of ME induction in association with H2O2 generation and the activity of two most important antioxidative enzymes (SOD and CAT) in isolated microspores of triticale and barley. The obtained data fully confirmed the hypothesis of ROS involvement in microspore reprogramming, revealing a strong positive correlation (r = 0.85) between H2O2 generation and ME effectiveness in conditions optimal for its initiation (Exp. 3).
Moreover, despite significant seasonal and genetic effects, pre-treatment of tillers with exogenously applied GSH, significantly influenced ELS development. Its effect was positive mainly when combined with mannitol (LT + MAN + GSH), which is standardly used for ME induction in barley, and can be considered as both a mild osmotic stress inducing factor, and as an osmoprotectant and a quencher of ROS (Meena et al. 2015). The combination of MAN and GSH treatments—although in a different time-sequence schedule—also induced a positive effect on ELS formation in androgenic cultures of rye (Zieliński et al. 2020). Positive effects of GSH were received earlier in several studies focused on somatic and androgenic embryo development, although GSH was usually applied directly to the medium at the stage of in vitro culture (Żur et al. 2019 and references therein). As previously revealed, the role of GSH is connected not only with antioxidative defense during microspore reprogramming but also with maintaining the intracellular redox balance regulating ELS development. In this case it is supposed that negative effects of GSH/OTC tiller pre-treatments observed in some cases were the result of excessive decomposition of H2O2, whose amount dropped below the threshold initiating microspore reprogramming.
A variation in the response to some tiller pre-treatments suggests that the threshold level of ROS required for microspore reprogramming is genotype-specific and significantly lower for barley in comparison with triticale microspores.
Comparing data received from the analyses of antioxidative enzymes activities in the two experiments, it was confirmed that high activity of SOD, which catalyzes the dismutation of superoxide anion to H2O2, was the first feature differentiating embryogenic microspore suspensions from the non-embryogenic ones (Fig. 7a). This conclusion was based on the comparison between responsive and recalcitrant DH lines/cultivars as well as on the comparison between results received for microspores isolated under optimal (Exp. 3) and non-optimal (Exp. 4) conditions for in vitro culture establishment. However, a high SOD activity is not the only prerequisite necessary for effective ME initiation, which could be seen in cv. Golden Promise in Exp. 4. It seems that a high generation of H2O2 resulting from high SOD activity also requires tools for its efficient decomposition. The negative correlation between CAT activity, microspore viability and embryogenic development shows only apparent discrepancy with this assumption, as a generally very low CAT activity was probably not sufficient to provide effective defense.
Summary. (a) The effect of low temperature (LT) tillers pre-treatment on reactive oxygen species (ROS) generation, the activity of the antioxidative system and the effectiveness of ME and its modulation induced by season × genotype interaction. (I) Depending on the physiological condition of donor plants, LT tillers pre-treatment induces ROS accumulation, which activates (responsive DH/cv.) or deactivates (recalcitrant DH/cv.) the antioxidative defence system. (II) Depending on the physiological condition of donor plants, isolation of microspores associated with excessive ROS generation results in: (A) high activity of superoxide dismutase (SOD) and catalase (CAT), intensive or moderate generation of hydrogen peroxide (H2O2) and effective induction of microspore embryogenesis (ME); (B) low activity of SOD, low generation of H2O2 and high frequency of viable microspores which continue gametophytic development; (C) low activity of SOD and CAT, moderate generation of H2O2 and high frequency of dead or damaged microspores. However, high activity of SOD and intensive generation of H2O2 are not the only prerequisites for effective ME induction, which could be seen on the example of cv. Golden Promise (D). V—microspore viability [%] determined by FDA assay; ME—effectiveness of ME expressed as the number of ELS per spike. A—embryogenic cultures of isolated microspores characterized by high frequency of star-like structures (triticale) and symmetrically divided microspores (barley). B—non-embryogenic cultures of isolated microspores characterized by high frequency of asymmetrically divided microspores. C—non-embryogenic cultures of isolated microspores characterized by high frequency of plasmolysed, shrinked microspores. Data are presented in colour according to an arbitrary adopted scale, with colour intensity corresponding to the values of enzyme activity and H2O2 generation intensity (Fig. 6). (b) The effect of different tillers pre-treatments on the effectiveness of ME and its modulation induced by season × genotype interaction. Data are presented in colour according to an arbitrary adopted scale (presented below), with colour intensity corresponding to the number of ELS developed per spike of the donor plant. Asterisk marks values statistically different from control (LT). GP—Golden Promise; LT—low-temperature tiller pre-treatment (21 days at 4 °C); LT + GSH—low-temperature tiller pre-treatment combined with the application of 0.3 mmol dm−3 reduced glutathione; LT + MAN—low-temperature tiller pre-treatment combined with the application of 0.3 mol dm−3 mannitol; LT + MAN + GSH—low-temperature tiller pre-treatment combined with the application of 0.3 mol dm−3 mannitol and 0.3 mmol dm−3 reduced glutathione; LT + DDC—low temperature tillers pre-treatment combined with the application of 0.25 mmol dm−3 diethyldithiocarbamate trihydrate; LT + OTC—low temperature tillers pre-treatment combined with the application of 0.25 mmol dm−3 l-2-oxothiazolidine-4-carboxylic acid. All chemicals were applied during the last 4 days before microspore isolation
The conducted experiments also revealed genetic specificity of antioxidative defense (Fig.7b). A relatively high H2O2 generation detected in isolated microspores of triticale in association with low activity of antioxidative enzymes (Exp. 4) resulted in extremely low cell viability, which was probably the primary cause of ME induction failure (Fig. 7a). In the case of barley, genotype specific low/high activity of SOD and H2O2 level (cv. Igri/cv. Golden Promise) associated with low activity of CAT did not diminish microspore viability but almost completely blocked embryogenic development in typically responsive isolated microspore cultures of cv. Igri. Different relationships between SOD and CAT in the tested lines may suggest that O2.−/H2O2 ratio also plays a role in ME induction, as both of these ROS have been indicated as signalling molecules (Dietz et al. 2016; Noctor et al. 2018; Mhamdi and van Breusegem 2018). The observed variation is in accordance with a well-known genomic specificity of plant cell response to ME-inducing treatment and suggests an important role of other enzymatic or non-enzymatic elements of antioxidative defense. It is known that H2O2 can be produced from O2.− not only through the reaction of disproportionation catalysed by SOD, but also through O2.− reduction by other reductants, like GSH or ascorbate. Similarly, CAT deficiency can be compensated by the activation of another enzymes—ascorbate or glutathione peroxidases, alternative oxidase or monodehydroascorbate reductase (Mittler 2002). Another almost non-recognized problem is subcellular and organellar specificity of ROS localization and scavenging and the question of how intracellular ROS distribution influences the interaction with other cellular messengers like PGRs, calcium ions or reactive nitrogen species (Rodriguez-Serrano et al. 2012; Zechmann 2014; Mignolet-Spruyt et al. 2016). Thus, further more detailed analyses of intercellular physiological background and its interaction with environmental stimuli are necessary for better understanding of the mechanism controlling successful ME initiation.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ari E, Bedir H, Yildirim S et al (2016) Androgenic responses of 64 ornamental pepper (Capsicum annuum L.) genotypes to shed-microspore culture in the autumn season. Turk J Biol 40(3):706–717. https://doi.org/10.3906/biy-1505-41
Arnaund D, Lee S, Takebayashi Y et al (2017) Cytokinin-mediated regulation of reactive oxygen species homeostasis modulates stomatal immunity in Arabidopsis. Plant Cell 29:543–559
Asif M, Eudes F, Goyal A et al (2013) Organelle antioxidants improve microspore embryogenesis in wheat and triticale. In Vitro Cell Dev Biol Plant 49(5):489–497. https://doi.org/10.1007/s11627-013-9514-z
Belmonte M, Stasolla C, Loukanina N et al (2003) Glutathione modulation of purine metabolism in cultured white spruce embryogenic tissue. Plant Sci 165(6):1377–1385. https://doi.org/10.1016/j.plantsci.2003.08.002
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Considine MJ, Foyer CH (2014) Redox regulation of plant development. Antioxid Redox Signal. https://doi.org/10.1089/ars.2013.5665
Coronado MJ, Hensel G, Broeders S et al (2005) Immature pollen-derived doubled haploid formation in barley cv. Golden Promise as a tool for transgene recombination. Acta Physiol Plant 27(4B):591–599. https://doi.org/10.1007/s11738-005-0063-x
Datta SK (2005) Androgenic haploids: factors controlling development and its application in crop improvement. Curr Sci 89(11):1870–1878
Dietz K-J, Mittler R, Noctor G (2016) Recent progress in understanding the role of reactive oxygen species in plant cell signaling. Plant Physiol 171(3):1535–1539. https://doi.org/10.1104/pp.16.00938
Dwivedi SL, Britt AB, Tripathi L et al (2015) Haploids: constraints and opportunities in plant breeding. Biotechnol Adv 33(6):812–829. https://doi.org/10.1016/j.biotechadv.2015.07.001
Earnshaw BA, Johnson MA (1985) The effect of glutathione on development in wild carrot suspension cultures. Biochem Biophys Res Commun 133(3):988–993. https://doi.org/10.1016/0006-291X(85)91233-1
Earnshaw BA, Johnson MA (1987) Control of wild carrot somatic embryo development by antioxidants. Plant Physiol 85(1):273–276. https://doi.org/10.1104/pp.85.1.273
Elhiti M, Yang C, Belmonte MF et al (2012) Transcriptional changes of antioxidant responses, hormone signalling and developmental processes evoked by the Brassica napus SHOOTMERISTEMLESS during in vitro embryogenesis. Plant Physiol Biochem 58:297–311. https://doi.org/10.1016/j.plaphy.2012.06.024
Ercan N, Sensoy FA, Sensoy S (2006) Influence of growing season and donor plant age on anther culture response of some pepper cultivars (Capsicum annuum L.). Sci Hortic Amsterdam 110(1):16–20. https://doi.org/10.1016/j.scienta.2006.06.007
Fernando VCD, Schroeder DF (2016) Shedding light on plant development: light signalling in the model plant Arabidopsis thaliana. Ceylon J Science 45(1):3–13. https://doi.org/10.4038/cjs.v45i1.7359
Franklin KA, Toledo-Ortiz G, Pyott GE et al (2014) Interaction of light and temperature signalling. J Exp Bot 65(11):2859–2871. https://doi.org/10.1093/jxb/eru059
Heslop-Harrison J, Heslop-Harrison Y (1970) Evaluation of pollen viability by enzymatically induced fluorescence: intracellular hydrolysis of fluorescein diacetate. Stain Technol 45(3):115–120. https://doi.org/10.3109/10520297009085351
Hoseini M, Ghadimzadeh M, Ahmadi B et al (2014) Effects of ascorbic acid, alpha-tocopherol, and glutathione on microspore embryogenesis in Brassica napus L. In Vitro Cell Dev Biol Plant 50(1):26–35. https://doi.org/10.1007/s11627-013-9579-8
Jacquard C, Asakaviciute R, Hamalian AM et al (2006) Barley anther culture: effects of annual cycle and spike position on microspore embryogenesis and albinism. Plant Cell Rep 25:375–381. https://doi.org/10.1007/s00299-005-0070-9
Jacquard C, Mazeyrat-Gourbeyre F, Devaux P et al (2009) Microspore embryogenesis in barley: anther pre-treatment stimulates plant defence gene expression. Planta 229(2):393–402. https://doi.org/10.1007/s00425-008-0838-6
Kocsy G, Tari I, Vanková R et al (2013) Redox control of plant growth and development. Plant Sci 211:77–91. https://doi.org/10.1016/j.plantsci.2013.07.004
Krzewska M, Czyczyło-Mysza I, Dubas E et al (2012) Quantitative trait loci associated with androgenic responsiveness in triticale (× Triticosecale Wittm.) anther culture. Plant Cell Rep 31(11):2099–2108. https://doi.org/10.1007/s00299-012-1320-2
Krzewska M, Czyczyło-Mysza I, Dubas E et al (2015) Identification of QTLs associated with albino plant formation and some new facts concerning green versus albino ratio determinants in triticale (x Triticosecale Wittm.) anther culture. Euphytica 206:263–227. https://doi.org/10.1007/s10681-015-1509-x
Kumlehn J, Serazetdinova L, Hensel G et al (2006) Genetic transformation of barley (Hordeum vulgare L.) via infection of androgenetic pollen cultures with Agrobacterium tumefaciens. Plant Biotechnol J 4(2):251–261. https://doi.org/10.1111/j.1467-7652.2005.00178.x
Larsen ET, Tuvesson IKD, Andersen SB (1991) Nuclear genes affecting percentage of green plants in barley (Hordeum vulgare L.) anther culture. Theor Appl Genet 82:417–420. https://doi.org/10.1007/BF00588593
Lee NY, Lee MJ, Kim YK et al (2010) Effect of light emitting diode radiation on antioxidant activity of barley leaf. J Korean Soc Appl Biol Chem 53:685–690. https://doi.org/10.3839/jksabc.2010.104
Lippmann R, Friedel S, Mock HP et al (2015) The low molecular weight fraction of compounds released from immature wheat pistils supports barley pollen embryogenesis. Front Plant Sci 6:498. https://doi.org/10.3389/fpls.2015.00498
Maraschin S, Caspers M, Potokina E et al (2006) cDNA array analysis of stress-induced gene expression in barley androgenesis. Physiol Plant 127(4):535–550. https://doi.org/10.1111/j.1399-3054.2006.00673.x
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244(22):6049–6055
Meena M, Prasad V, Zehra A et al (2015) Mannitol metabolism during pathogenic fungal–host interactions under stressed conditions. Front Microbiol 6:1019. https://doi.org/10.3389/fmicb.2015.01019
Mhamdi A, van Breusegem F (2018) Reactive oxygen species in plant development. Development 145(9):dev164376. https://doi.org/10.1242/dev.164376
Mignolet-Spruyt L, Xu E, Idänheimo N et al (2016) Spreading the news: subcellular and organellar reactive oxygen species production and signaling. J Exp Bot 67(13):3831–3844. https://doi.org/10.1093/jxb/erw080
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410. https://doi.org/10.1016/s1360-1385(02)02312-9
Mittler R (2017) ROS are good. Trends Plant Sci 22(1):11–19. https://doi.org/10.1016/j.tplants.2016.08.002
Muñoz-Amatriaín M, Svensson J, Castillo A et al (2006) Transcriptome analysis of barley anthers: effect of mannitol treatment on microspore embryogenesis. Physiol Plant 127(4):551–560. https://doi.org/10.1111/j.1399-3054.2006.00729.x
Nguyen V, Fleury D, Timmins A et al (2015) Addition of rye chromosome 4R to wheat increases anther length and pollen grain number. Theor Appl Genet 128:953–964. https://doi.org/10.1007/s00122-015-2482-4
Noctor G, Reichheld JP, Foyer CH (2018) ROS-related redox regulation and signaling in plants. Semin Cell Dev Biol 80:3–12. https://doi.org/10.1016/j.semcdb.2017.07.013
Otto I, Müller A, Kumlehn J (2015) Barley (Hordeum vulgare L.) transformation using embryogenic pollen cultures. In: Wang K (ed) Agrobacterium protocols, Methods in molecular biology, 1223, vol 1. Springer, New York, pp 85–99
Pauk J, Mhaly R, Monostori T et al (2003) Protocol for triticale (× Triticosecale Wittmack) microspore culture. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) Doubled haploid production in crop plants. A manual. Kluwer, Dordrecht, pp 129–134
Ritala A, Mannonen L, Oksman-Caldentey KM (2001) Factors affecting the regeneration capacity of isolated barley microspores (Hordeum vulgare L.). Plant Cell Rep 20:403–407. https://doi.org/10.1007/s002990100345
Rodriguez-Serrano M, Bárány I, Prem D et al (2012) NO, ROS, and cell death associated with caspase-like activity increase in stress-induced microspore embryogenesis of barley. J Exp Bot 63(5):2007–2024. https://doi.org/10.1093/jxb/err400
Schmitt FJ, Renger G, Friedrich T et al (2014) Reactive oxygen species: re-evaluation of generation, monitoring and role in stress-signaling in phototrophic organisms. Biochim Biophys Acta 1837(6):835–848
Shariatpanahi ME, Bal U, Heberle-Bors E et al (2006) Stresses applied for the re-programming of plant microspores towards in vitro embryogenesis. Physiol Plant 127(4):519–534. https://doi.org/10.1111/j.1399-3054.2006.00675.x
Silva T (2010) Indica rice anther culture: can the impasse be surpassed? Plant Cell Tissue Organ Cult 100(1):1–11. https://doi.org/10.1007/s11240-009-9616-9
Sinha RK, Eudes F (2015) Dimethyl tyrosine conjugated peptide prevents oxidative damage and death of triticale and wheat microspores. Plant Cell Tissue Organ Cult 122:227–237. https://doi.org/10.1007/s11240-015-0763-x
Stasolla C, Belmonte MF, van Zyl L et al (2004) The effect of reduced glutathione on morphology and gene expression of white spruce (Picea glauca) somatic embryos. J Exp Bot 55(397):695–709. https://doi.org/10.1093/jxb/erh074
Stasolla C, Belmonte MF, Tahir M et al (2008) Buthionine sulfoximine (BSO)-mediated improvement in cultured embryo quality in vitro entails changes in ascorbate metabolism, meristem development and embryo maturation. Planta 228(2):255–272. https://doi.org/10.1007/s00425-008-0735-z
Testillano PS (2019) Microspore embryogenesis: targeting the determinant factors of stress-induced cell reprogramming for crop improvement. J Exp Bot 70(11):2965–2978. https://doi.org/10.1093/jxb/ery464
Tilley MS, Heiniger RW, Crozier CR (2019) Tiller initiation and its effects on yield and yield components in winter wheat. Agron J 111(3):1–10. https://doi.org/10.2134/agronj2018.07.0469
Toldi D, Gyugos M, Darkó É et al (2019) Light intensity and spectrum affect metabolism of glutathione and amino acids at transcriptional level. PLoS ONE 14(12):e0227271. https://doi.org/10.1371/journal.pone.0227271
Uváčková L, Takáč T, Boehm N et al (2012) Proteomic and biochemical analysis of maize anthers after cold pretreatment and induction of androgenesis reveals an important role of anti-oxidative enzymes. J Proteome 75(6):1886–1894. https://doi.org/10.1016/j.jprot.2011.12.033
Vrinten PL, Nakamura T, Kasha KJ (1999) Characterization of cDNAs expressed in early stages of microspore embryogenesis in barley (Hoedeum vulgare) L. Plant Mol Biol 41(4):455–465. https://doi.org/10.1023/A:1006383724443
Wang M, van Bergen S, van Duijn B (2000) Insights into a key developmental switch and its importance for efficient plant breeding. Plant Physiol 124(2):523–530. https://doi.org/10.1104/pp.124.2.523
Wędzony M (2003) Protocol for anther culture in hexaploid triticale (x Triticosecale Wittm.). In: Maluszynski M, Kasha KJ, Forster BP et al (eds) Doubled haploid production in crop plants—a manual. Kluwer, Dordrecht/Boston/London, pp 123–128
Xia XJ, Zhou YH, Shi K et al (2015) Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J Exp Bot 66(10):2839–2856. https://doi.org/10.1093/jxb/erv089
Yeung EC, Belmonte MF, Tu LTT et al (2005) Glutathione modulation of in vitro development. In Vitro Cell Dev Biol Plant 41:584–590. https://doi.org/10.1079/IVP2005683
Zechmann B (2014) Compartment-specific importance of glutathione during abiotic and biotic stress. Front Plant Sci 5:556. https://doi.org/10.3389/fpls.2014.00566
Zeng A, Song L, Cui Y et al (2017) Reduced ascorbate and reduced glutathione improve embryogenesis in broccoli microspore culture. S Afr J Bot 109:275–280. https://doi.org/10.1016/j.sajb.2017.01.005
Zhuang JJ, Xu J (1983) Increasing differentiation frequencies in wheat pollen callus. In: Hu H, Vega MR (eds) Cell and tissue culture techniques for cereal crop improvement. Science Press, Beijing, p 431
Zieliński K, Krzewska M, Żur I et al (2020) The effect of glutathione and mannitol on androgenesis in anther and isolated microspore cultures of rye (Secale cereale L.). Plant Cell Tissue Organ Cult 140:577–592. https://doi.org/10.1007/s11240-019-01754-9
Żur I, Dubas E, Golemiec E et al (2008) Stress-induced changes important for effective androgenic induction in isolated microspore culture of triticale (× Triticosecale Wittm.). Plant Cell Tissue Organ Cult 94(3):319–328. https://doi.org/10.1007/s11240-008-9360-6
Żur I, Dubas E, Golemiec E et al (2009) Stress-related variation in antioxidative enzymes activity and cell metabolism efficiency associated with embryogenesis induction in isolated microspore culture of triticale (x Triticosecale Wittm.). Plant Cell Rep 28(8):1279–1287. https://doi.org/10.1007/s00299-009-0730-2
Żur I, Krzewska M, Dubas E et al (2012) Molecular mapping of loci associated with abscisic acid accumulation in triticale (× Triticosecale Wittm.) anthers in response to low temperature stress inducing androgenic development. Plant Growth Regul 68(3):483–492
Żur I, Dubas E, Krzewska M et al (2014) Antioxidant activity and ROS tolerance in triticale (× Triticosecale Wittm.) anthers affect the efficiency of microspore embryogenesis. Plant Cell Tissue Organ Cult 119(1):79–94. https://doi.org/10.1007/s11240-014-0515-3
Żur I, Dubas E, Krzewska M et al (2015) Hormonal requirements for effective induction of microspore embryogenesis in triticale (× Triticosecale Wittm.) anther cultures. Plant Cell Rep 34(1):47–62. https://doi.org/10.1007/s00299-014-1686-4
Żur I, Dubas E, Krzewska M et al (2019) Glutathione provides antioxidative defence and promotes microspore-derived embryo development in isolated microspore cultures of triticale (x Triticosecale Wittm.). Plant Cell Rep 38(1):195–209. https://doi.org/10.1007/s00299-018-2362-x
Acknowledgements
The seeds of DH lines of triticale were received from the State Breeding Institute at the University of Hohenheim (Germany) thanks to the kindness of Dr. H.P. Maurer. We would also like to thank to Dr. Jochen Kumlehn from the Leibniz Institute of Plant Genetics and Crop Plant Research (Gatersleben, Germany) for the seeds of two barley cultivars, ‘Igri’ and ‘Golden Promise’. The study was conducted as a part of a research project 2015/18/M/NZ3/00348 financed by the National Science Center, Poland.
Funding
The study was funded from Polish National Project 2015/18/M/NZ3/00348 financed by National Science Centre.
Author information
Authors and Affiliations
Contributions
IŻ conceived and designed the research, conducted experiments and wrote the manuscript. ED, MK, AN, KJ and SM performed experiments. PK and ES estimated H2O2 generation, KG and GG measured the activity of antioxidative enzymes. ED prepared photographic images. All authors read, reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Klaus Eimert.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Table 1
(DOCX 17 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Żur, I., Dubas, E., Krzewska, M. et al. Triticale and barley microspore embryogenesis induction requires both reactive oxygen species generation and efficient system of antioxidative defence. Plant Cell Tiss Organ Cult 145, 347–366 (2021). https://doi.org/10.1007/s11240-021-02012-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-021-02012-7