Abstract
The present study was an attempt to develop an in vitro colchicine chromosome doubling protocol to restore the fertility of an F1 interspecific hybrid in Lilium. Basal scale segments of Lilium × formolongi × Oriental hybrid (FO) bulblets were pre-cultured for three durations (6, 15 and 25 days) and soaked in three colchicine concentrations (0.00, 1.25 and 2.50 mM) for 18, 24 and 36 h. To separate mixoploids, three cycles of adventitious bud induction were performed. The ploidy levels of the surviving plantlets were detected by flow cytometry at 30–31 weeks after induction and were confirmed by chromosome counts. The results indicated that the pre-culture duration, colchicine concentration and exposure time all had significant impacts on the tetraploid induction rate. The preferred procedure was to pre-culture the segments for 6 days and then treat them with 1.25 mM colchicine for 24 h. The morphological traits of rosette leaves were significantly different between the tetraploid and diploid plants. The adult tetraploid plants had considerably longer and wider leaves, larger flowers, and delayed flowering time (8 days later) than did diploid plants. Pollen viability tests and backcross trials of FO hybrids demonstrated fertility restoration at the tetraploid level. This protocol provides a feasible method for inducing fertile tetraploid FO hybrids for further breeding.
Similar content being viewed by others
Introduction
The lily is one of the most economically important monocot flower bulbs worldwide and is primarily used as a cut flower and pot plant. For lily breeding, interspecific hybridization plays an important role (Van Tuyl and Lim 2003). To date, a range of interspecific hybrids, including L. longiflorum × Asiatic hybrids (LA), L. longiflorum × Oriental hybrids (LO), Oriental hybrids × Asiatic hybrids (OA), Oriental hybrids × Trumpet hybrids (OT) and L. auratum × L. henryi (AuH), have been bred successfully (Barba-Gonzalez et al. 2014). However, most of these hybrids, including Oriental hybrids, take 2–4 years to grow from seed to flower (Bakhshaie et al. 2016).
Lilium× formolongi was obtained from repeated interspecific crosses of L. formosanum × L. longiflorum, which flowers within 1 year from seeds and has frost tolerance and winter hardiness (Okazaki 1996; Anderson et al. 2009). L. × formolongi × Oriental hybrids (FO) are new hybrids that were produced with the aim of transferring desirable horticultural traits from L. × formolongi to Oriental hybrids (Rhee and Kim 2008). Our research group also successfully developed a diploid FO hybrid through interspecific crossing between L. × formolongi ‘Raizan 3’ (2n = 2x = 24) and the Oriental hybrid ‘Sorbonne’ (2n = 2x = 24) using cut-style pollination and the embryo rescue technique. The hybrids exhibited heterosis, including early flowering, rapid growth and strong resistance, but the flower shape and upward direction are undesirable. Therefore, repetitive backcrossing is the obligatory path for new cultivars. However, it is difficult for this FO hybrid to produce progeny as a male or female. The same fertility problem was faced during the other interspecific hybridization of this genus (Van Tuyl and Van Holsteijn 1996). Thus, to overcome such problems, these hybrids must be converted into tetraploids.
Mitotic polyploidization have been commonly used for polyploid breeding (Van Tuyl et al. 2000; Takamura et al. 2002; Chandanie et al. 2011) and to restore the fertility of interspecific F1 hybrids in Lilium (Van Tuyl et al. 1990, 1992; Lim et al. 2000, 2002; Han and Niimi 2008; Barba-Gonzalez et al. 2014). In an in vitro chromosome doubling process, the optimal antimitotic agent, its concentration and the exposure time required for successful polyploidization are species-dependent (Dhooghe et al. 2011). In previous studies, polyploid induction in lilies was achieved by using various explants, particularly in vitro stock bulblet scales or bulblet scale segments because of their sensitive morphological responses and high regenerative ability (Bakhshaie et al. 2010; Bi et al. 2015). However, the optimal bulblet size, scale position and application method that provide the highest shoot regeneration for chromosome doubling have never been identified. Therefore, a low efficiency of polyploid regeneration production has been observed, along with the production of mixoploids (Van Tuyl et al. 1992; Takamura et al. 2002; Chandanie et al. 2011).
Colchicine is the most commonly used antimitotic agent for in vitro chromosome doubling. However, despite its high affinity for the microtubules of animal cells, it binds poorly to plant tubulins. Thus, it must be used in relatively high concentrations that are toxic to plants and inhibit plant regeneration (Dhooghe et al. 2011). These drawbacks encouraged searches for alternative antimitotic agents, and colchicine sterilization methods are worth considering. Zhang et al. (2007) compared the effect of filter-sterilized and autoclaving methods for colchicine on regeneration and tetraploid induction in Citrus sinensis; the result showed that autoclaved colchicine was more effective. In lily polyploidization, only one report mentioned the colchicine sterilization method, and the filter-sterilized method for in vitro chromosome doubling was instead employed (Han and Niimi 2008). In this study, we attempt to use the autoclaved colchicine method.
Previous studies reported that a certain number of mixoploids were associated with the process of tetraploid induction (Roy et al. 2001; Regalado et al. 2015). This phenomenon was also widely present in Lilium (Van Tuyl et al. 1990; Takamura et al. 2002; Chandanie et al. 2011). However, efficient methods to separate mixoploid lily plants are still lacking.
The objective of the present study was to develop an optimized procedure for inducing tetraploidy to restore the fertility of the FO hybrid. Phenotypic characters and fertility between diploid and tetraploid plants are also described.
Materials and methods
Plant material and culture conditions
Interspecific hybrids between L. × formolongi ‘Raizan 3’ (2n = 2x = 24) and the Oriental hybrid ‘Sorbonne’ (2n = 2x = 24) were obtained using a cut-style pollination and embryo rescue technique during the immature stage. The hybrid plantlets were cultured for at least 4 months in the dark on bulblet formation medium, and the basal part (0.8–1.0 cm wide) of the external and middle scales was excised from the bulblets (1.5–1.8 cm in diameter) were used as the starting explants for pre-culturing. These explants were initially cultured on adventitious shoot induction medium, which was composed of Murashige and Skoog (1962) (MS) basal salts medium supplemented with 2.0 mg l−1 6-benzylaminopurine (6-BA), 0.1 mg l−1 1-naphthaleneacetic acid (NAA), 30 g l−1 sucrose and 7 g l−1 agar at pH 5.8. Unless otherwise stated, all in vitro cultures were maintained at 22 ± 2 °C under a 16-h photoperiod at light intensity of 45 μmol m−2 s−1. The materials after 6, 15 and 25 days of pre-culture (Fig. 1a–c) were taken as explants in the following experiments.
In vitro chromosome doubling
Stock solutions of colchicine (1 g of colchicine in 2 ml of DMSO) were prepared and diluted with sterilized distilled water for working solutions at 1.25 and 2.50 mM and autoclaved. This colchicine was used for the induction experiment. Sterilized distilled water was used as a control (0 mM colchicine).The basal scale segments for each pre-culture duration were immersed in the colchicine solution for 18, 24 and 36 h, respectively. The orthogonal table of L 9 (34) was selected to investigate the three factors’ effects on the tetraploid induction rate (Table 1). Nine treatments were arranged according to the design, and the whole experiment was repeated three times with ten explants per treatment.
After the colchicine treatment was rinsed with sterilized distilled water, the explants were transferred to fresh adventitious shoot induction medium for 6 weeks. Then, single shoots (0.5–1 cm in length) were isolated and transferred to the same induction medium twice with 6 weeks’ culture to separate mixoploids. After three cycles of adventitious shoot induction, single shoots (0.5–4 cm in length) from different treatments were isolated and transferred to a development medium for 6 weeks, in which the 6-BA concentration was reduced to 1.0 mg l−1. Finally, single plantlets were isolated and sub-cultured in the dark for 6 weeks on bulblet formation medium, which contained MS basal salts medium supplemented with 80 g l−1 sucrose, 7 g l−1 agar and 1 g l−1 activated charcoal.
Flow cytometry (FCM) analysis of ploidy level
The procedure for ploidy analysis was slightly modified from a previous protocol (Doležel et al. 2007). To release nuclei, 150 mg of young leaves was placed in a Petri dish containing 1.5 ml of extraction buffer (0.1 M citric acid containing 0.5% Tween-20) and were finely chopped using a razor blade. The homogenate was kept at room temperature for 5 min and then filtered through a 30-μm nylon mesh. The supernatant was removed, and 200 μl of 1 μg ml−1 propidium iodide (PI) staining buffer was added. FCM was conducted after the nuclei had been incubated for 25 min at 4 °C. Histograms were generated after analyzing ≥5000 nuclei using the FSC 3.0 Flow-Cytometry Express software. The experiment was repeated three times to confirm reproducibility.
Chromosomal counts
Single bulblets were transferred to MS basal salts medium supplemented with 0.2 mg l−1 NAA, 30 g l−1 sucrose and 7 g l−1 agar to the roots. Chromosome were squashed according to the technique by Zhou (2007).
Evaluation of phenotypic characters
The diploid and selected tetraploid bulblets (1–2 cm in diameter) were transferred to cold conditions (4 °C) to break dormancy. After 9 weeks of storage, 150 bulblets each of the diploid and tetraploid were transplanted to soil and grown from 14 September 2015 in a greenhouse at Beijing Forestry University. Sixty representative samples of the diploids and tetraploids were randomly chosen to compare morphological traits at both the juvenile and adult stages. The seventh fully expanded rosette leaves were observed after 4 months of cultivation. Leaf lengths and widths were measured with a ruler, leaf thicknesses were measured using Vernier calipers and the leaf index was calculated as the leaf length : width ratio. To evaluate the stomata index and chloroplast numbers, stomata were stained with 1% I2-KI. The first flower opening times of adult diploid and tetraploid plants were 15 June 2016 and 23 June 2016, respectively. At the full-bloom stage, plants were randomly selected to compare plant heights, leaf lengths, leaf widths, flower sizes and stem diameters. For each plant, the lengths and widths of the three leaves in the middle of the stem were measured. The number of internodes was counted between two nodes from the plant root to the tip. Ananalysis of variance (ANOVA) was performed, and differences among treatments were considered statistically significant at p ≤ 0.05 using Duncan’s multiple range tests. The results were expressed as the means ± standard error (SE). Statistical analyses were performed using the statistical software SPSS version 19.0.
Karyotype analysis by GISH and FISH
The GISH and FISH procedures were basically the same as those described by Barba-Gonzalez et al. (2005). GISH and FISH were performed sequentially on the same preparations. Sonicated genomic DNA (1–10 kb) from L. × formolongi ‘Raizan 3’ was used as a probe after being labeled with digoxigenin-16-dUTP by nick translation (Dig-Nick Translation Mix, Roche, Germany). Autoclaved DNA (100–500 bp) from the Oriental hybrid ‘Siberia’ was used to block the non-hybridized sequences, and the 45S rDNA was labeled with biotin-11-dUTP by nick translation. For rehybridization, the genomic probe was removed using the method of Schwarzacher and Heslop-Harrison (2000). Fluorescence signals were detected with a fluorescence microscope(OLYMPUS BX51, Japan). Images were captured with a charge-coupled device system and processed using the Adobe Photoshop CS6 software. At least ten images of mitotic metaphase chromosomes were measured using the computer software Computer Aided Design. The chromosomes were arranged based on decreasing short arm lengths as in Lim et al. (2001).
Pollen viability observation
To measure the pollen stainability, a mixed sample of pollen from two to three anthers of each plant analyzed was stained with 2% aceto-carmine and viewed under the microscope. In vitro germination of pollen was carried out at 25 °C for 8 h under darkness on an artificial liquid medium containing 80 g l−1 sucrose, 50 mg l−1 boric acid and 40 mg l−1 calcium chloride, adjusted to pH 5.8. Three randomly selected microscopic field areas each were observed for 30 samples of diploid and tetraploid plants.
Backcrosses
Reciprocal crosses were conducted between tetraploid FO hybrids and their parents, Oriental hybrid ‘Sorbonne’ and L. × formolongi (Table 7). After pollination, the capsules and seeds with embryos were investigated. The backcross seedlings were obtained through the culture on seed germination medium.
Results
Effects of pre-culture duration, colchicine concentration and exposure time on tetraploid induction rate
According to the L 9(34) orthogonal table, nine treatments are present in Table 1.The ploidy levels of the 992 surviving plantlets were determined by FCM at 30–31 weeks after induction and were confirmed by chromosome counts. In total, 192 tetraploids were obtained, as were five chimeras and three octoploids, which were excluded from data collection. The representative histogram shows that the tetraploids were doubles of their corresponding diploids (Fig. 3a, b). The chromosome numbers of the diploid plantlets were 2n = 2x = 24, and those of the tetraploids were 2n = 4x = 48 (Fig. 3c, d). The chromosomal counts were significantly correlated with the FCM analysis.
According to the tetraploid induction rate data (Table 1). GLM-univariate analysis (Table 2) indicated that the tetraploid induction rate was significantly influenced by the pre-culture duration (F = 12.438, p = 0.000), colchicine concentration (F = 30.841, p = 0.000) and exposure time (F = 12.677, p = 0.000).
Different pre-culture durations, colchicine concentrations and exposure times had different effects on the tetraploid induction rate (Table 3). As shown in Table 3, the tetraploid induction rates were significantly different among the 6-(31.95 ± 9.08), 15-(9.20 ± 3.23) and 25-day (20.83 ± 6.80) groups, with the 6-day pre-culture duration exhibiting the most positive effect. No tetraploid plantlets were induced when segments were treated with 0.00 mM colchicine. There were no significant differences in the tetraploid induction rates between 1.25 mM colchicine (30.15 ± 6.00) and 2.50 mM colchicine (31.82 ± 7.45). Considering the economic benefit of more dilute solutions, 1.25 mM colchicine is preferred. Moreover, a significantly higher tetraploid induction rate was obtained after 24 h of exposure (31.55 ± 8.46) than after 36 h (21.77 ± 7.46) or 18 h (8.66 ± 3.38) of exposure. Therefore, the preferred combination for inducing tetraploids was to pre-culture the basal scale segments for 6 days and then treat them with 1.25 mM colchicine for 24 h.
The first visible effect of colchicine was delayed growth. Colchicine-treated plantlets grew more slowly than control plantlets, and their leaves were more fleshy and rough (Fig. 2a, b). Additionally, abnormal growth, including callogenesis, decomposed shoot apices, plantlet yellowing and delayed root induction with slow growth, was observed (Fig. 2c–f).
Normal and abnormal plantlets regenerated from basal scale segments. a Healthy shoot regeneration from scale explants 50 days after immersion in sterilized distilled water. b Healthy shoot regeneration from scale explants 80 days after immersion in colchicine solution. c Scale callogenesis. d Decomposed shoot apices. e Plantlet yellowing. f Delayed root induction with slow growth
Morphological traits of rosette leaves
After 4 months of growth in a greenhouse, the rosette leaves of the tetraploids were wider and shorter, resulting in a lower leaf length to width ratio compared with the diploids (Table 4). Notably, the leaves of tetraploid plants were greener than were those of the diploids and had thicker uneven surfaces and upward architecture (Fig. 3e, f). Stomatal densities, stomatal lengths and chloroplast numbers were also compared between the diploids and tetraploids. The results showed that there were significant variations (Table 4). Compared with the diploids, the stomatal density was dramatically reduced in the tetraploids (Fig. 4a, b). The average stomatal length was 104.53 ± 0.87 μm in the tetraploid plantlets but only 69.94 ± 0.51 μm in the diploids (Fig. 4c, d). Although determining the exact chloroplast number in two guard cells of the stomata was difficult, the numbers in tetraploid plants were greater than were the numbers in diploids (Fig. 4e, f).
Comparison of diploids (top panels) and tetraploids (bottom panels). a, b Flow cytometry analysis of the relative DNA amounts of PI-stained nuclei from fresh young leaves. The x- and y-axes represent the relative signal intensity and number of nuclei, respectively. The diploid (a) was used as a control standard to compare the ploidy levels of tetraploid (b) plants. c, d Chromosomes of a root tip cell from diploid 2n = 2x = 24 (c) and induced tetraploid 2n = 4x = 48 (d) FO hybrids; bars 10 μm. e, f Plants were planted in a greenhouse for 4 months after in vitro culture
Comparison of diploids (top panels) and tetraploids (bottom panels). a, b Stomatal densities in abaxial leaf epidermis. c, d Stomata lengths. e Chloroplast number within guard cells under 100× magnification. f Chloroplast number within guard cells under 63× magnification. Bars 100 μm (a, b); 20 μm (c, d, f); 10 μm (e)
Karyotype analysis by GISH and FISH
In situ hybridization was employed to analyze the karyotypic stability of tetraploid plants. Ploidy levels, the numbers of chromosomes from the parental genomes and 45S rDNA sites were clearly detected with GISH and FISH (Fig. 5a, b). In the diploid plants, 12 chromosomes of the L. × formolongi (F) genome and 12 chromosomes of the Oriental (O) genome were identified (Fig. 5a). In the diploids, there were four 45S rDNA sites on the chromosomes. One 45S rDNA site originating from the O genome was located in the short arm of chromosome 7 in close proximity to the centromere. The remaining three sites were located in F-genome chromosomes; one was located in the short arm of chromosome 17, and the other two were located in the terminal and pericentromeric regions of the long arms of chromosomes 5 and 8, respectively (Fig. 5c). The tetraploid plants had twice as many chromosomes as the diploids—24 chromosomes each of the F and O genomes—and eight 45S rDNA sites localized at the same chromosomal positions (Fig. 5b, d). The chromosomes of the diploids and tetraploids had very similar morphologies (Fig. 5c, d), and no chromosomal rearrangements were observed in the tetraploid plants. Thus, the tetraploids possessed karyotypic stability, as did the diploids.
Chromosome composition identified by genomic in situ hybridization and 45S rDNA site detection. In all cases, pink fluorescence represents L. × formolongi ‘Raizan 3’ chromosomes (F), and blue fluorescence represents ‘Sorbonne’ chromosomes (O). Chromosomes with 45S rDNA showed green fluorescence. a, b GISH and FISH on mitotic metaphase chromosomes (diploid and tetraploid); bars 10 μm. c, d Ideograms of diploid and tetraploid chromosomes. (Color figure online)
Phenotypic characters of adult plants
During the first growth season in the greenhouse, there were no significant differences in growth between diploids and tetraploids based on visual observation. The diploid and tetraploid bulblets (1–2 cm in diameter) took 275 and 283 days to bloom. Flowering was delayed by 8 days in the tetraploid plants compared with the diploids. No evident differences were identified in first flower opening time within the diploid or tetraploid groups. However, the tetraploids had a lower flowering percentage than did the diploids (85 and 95%, respectively). Evident differences were observed in the general appearance between the diploids and corresponding tetraploids (Table 5; Fig. 6a). The average flower size of the tetraploid plantlets (17.83 ± 0.72 cm) was significantly higher than that of the diploids (14.71 ± 0.58 cm). Thus, the tetraploid plants had considerably larger flowers (Fig. 6b). There were no significant differences in plant height or stem diameter between the diploids and tetraploids (Table 5). Both the number of flowers per plant and the number of leaves per plant were significantly reduced in tetraploid plants. However, there were no differences in the number of internodes per plant. The average leaf length was 17.75 ± 0.50 cm in tetraploid plants but only 15.87 ± 0.42 cm in the diploids. The average leaf width was 3.87 ± 0.13 cm in tetraploid plants and 3.43 ± 0.13 cm in the corresponding diploids. Therefore, tetraploid plants had much longer and wider leaves than did the diploid ones (Table 5; Fig. 6c).
Pollen viability and backcrosses
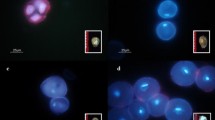
In the aceto-carmine staining test, 0% of the diploid pollen grains were stained, whereas 3097 (73.2%) of the 4230 tetraploid pollen grains were strongly stained red (Table 6; Fig. 7a, b), thus indicating the range of pollen fertility. In pollen germination on artificial liquid medium, 0% of the diploid pollen grains germinated, whereas 757 (52.6%) of the 1440 tetraploid pollen grains germinated (Table 6; Fig. 7c, d). The results of reciprocal crosses between tetraploid FO hybrids with Oriental hybrid ‘Sorbonne’ and L. × formolongi are shown in Table 7. When the tetraploids were used as the male parent, 256 seedlings were obtained from 74 capsules. The 4x × 2x crosses were rarely successful, and only three seedlings developed from 32 capsules. Therefore, tetraploid plants (FFOO) were more suitable to obtain BC1 hybrids as male than as female. In conclusion, the fertility of diploid FO hybrids was restored at the tetraploid level.
Pollen stainability and germination test. a Diploid pollen grains were shrunken and unstained by aceto-carmine (black arrow). b Tetraploid pollen grains were large and strongly stained red by aceto-carmine (red arrow). c Diploid pollen grains did not germinate on artificial liquid medium. d Tetraploid pollen grains germinated on an artificial liquid medium. Bars 100 μm (a, b, c, d)
Discussion
The production of interspecific L. × formolongi × Oriental hybrid (FO) hybrids was sought for introgression of L. × formolongi chromosomes into Oriental hybrids (Rhee and Kim 2008). To eliminate undesirable traits, backcrosses were inevitable. However, the F1 FO hybrids were sterile. The most widely used method for restoring fertility in interspecific hybrids is to obtain a tetraploid plant by doubling the chromosome number of the F1 hybrids (Lim et al. 2000).
A suitable explant type is an important factor that influences the success of polyploid induction (Dhooghe et al. 2011). Takayama and Misawa (1980) reported that bulblet size and position are important factors that influence the organ differentiation of Lilium. Hu (2007) developed a highly efficient procedure for the shoot regeneration of Lilium using basal scale segment explants. However, this procedure has not yet been used for the induction of lily tetraploids. Our preliminary experiments showed that the bulblet size and scale position of the FO hybrid affected the induction efficiency and survival rates. When the bulblets reached diameters of 1.5–1.8 cm after at least 4 months of culture, the basal parts (0.8–1.0 cm wide) of the external and middle scales as explants showed a higher regeneration rate and a certain resistance to colchicine toxicity. Moreover, the direct immersion of scale segments in colchicine solution resulted in low induction rates. Therefore, to increase the induction rates, the application method of explants is crucial. Commonly, an in vitro pre-incubation period is used to synchronize mitotic divisions before the antimitotic agent is applied and thereby ameliorate the effect of the antimitotic agent (Dhooghe et al. 2011). However, the optimal pre-culture durations depend on plant materials and explant types. In Populus, leaves were pre-cultured for 6 days (Xu et al. 2016). In C. sinensis, calli were pre-cultured for 6 days (Zhang et al. 2007). In Syringa vulgaris × S. pinnatifolia hybrids, nodal sections were pre-cultured for 5 days (Rose et al. 2000), and in cotton seeds, they were pre-cultured for 24–30 h (Omran and Mohammad 2008). The present study showed that when basal scale segments were pre-cultured for 6 days, a considerable number of tetraploids was produced. This result is consistent with histological observations of basal leaf segments of the Oriental hybrid ‘Siberia’ during shoot regeneration (Yin et al. 2013) and the bulblet morphogenesis of L. × formolongi in scale propagation (Ning et al. 2003). In both of those studies, the dividing cells formed typical meristemoids on day 6 of culture and underwent vigorous cell division.
The colchicine that is used in most published reports is filter-sterilized (Roy et al. 2001; Dhooghe et al. 2009; Yan et al. 2016), although in some studies, it is added to media before autoclaving (Gomes et al. 2014). Zhang et al. (2007) reported that autoclaving the colchicine solution did not reduce its polyploidization capacity but did alleviate the toxicity to meristematic tissue in C. sinensis. Thus, autoclaved colchicine was chosen in our in vitro chromosome doubling procedure. Colchicine-treated explants grew more slowly than did control explants at the initial culture stage, and few plants showing lethality, abnormal growth and failure to root were observed. The slow growth may have been due to the inhibitory effect of colchicine on cell division. However, after multiple sub-cultures (one cycle induction culture), both diploid and tetraploid plantlets grew equally well, which suggests that colchicine caused only an initial retardation of growth, as observed in Pogostemon cablin (Yan et al. 2016). Furthermore, GISH and FISH karyotype analysis suggested no chromosomal variation; the tetraploids showed twice the number of karyological traits observed in the diploids (Anssour et al. 2009; Gomes et al. 2014).
Occasionally, chromosome doubling resulted in mixoploids with different ploidy levels in different plant cells or organs (Van Tuyl et al. 1990; Rose et al. 2000; Dhooghe et al. 2009; Gomes et al. 2014; Xu et al. 2016). To date, there are three published protocols describing the separation of the components of a mixoploid. In Humulus lupulus, a complicated technique included a step of callus proliferation from the mixoploid shoots and the regeneration of new shoots with different ploidy (Roy et al. 2001). In Asparagus officinalis, the mixoploid plants detected through flow cytometry were dissected in different parts, leaving a single shoot with a root in each explant (Regalado et al. 2015). In Pyrus communis, the efficient method of separating mixoploid plants into pure types relied on adventitious shoot regeneration; two months later, the regenerants were evaluated for their chimeral status (Abu-Qaoud et al. 1990). In our study, three cycles of adventitious bud induction with a 6-week interval were used to separate mixoploids; the process of separation was considered successful because only five mixoploids were identified among the 200 polyploid plantlets.
Several different F1 interspecific lily hybrids (LH, LA, HC, LR, LO and OA) show absolute sterility, but fertility has been restored through mitotic chromosome doubling (Barba-Gonzalez et al. 2014). Lim et al. (2000) found that the pollen fertility of the amphidiploid (LLRR) hybrids was up to 40–50% stainability and 20% germination. Rhee and Kim (2008) proved that the pollen fertility of the amphidiploid (FFAA) was approximately 40% germination. Both the LLRR and FFAA pollen were successfully used for backcrossing. Our results also showed a high percentage of pollen staining (73.2%) and germination (52.6%) at the tetraploid level, which was slightly higher than those observed in the amphidiploid LLRR and FFAA hybrids. Differences in the range of pollen viability may be influenced by genotype differences, coloring agents and germination medium. The results of the tetraploid crosses with their parents proved the value of this protocol. For the reciprocal crosses, seed production was different, which showed that the incompatibility still existed. However, for the unsuccessful crosses of 4x–2x, this is possibly because the ploidy level of the endosperm nucleus (8x) produced by the tetraploid is too high because the phenomenon occurred between AA × LALA (2x–4x) and LALA × AA (4x–2x) in the study by Zhou (2007).
In conclusion, we have successfully developed an in vitro chromosome doubling protocol for the fertility restoration of the sterile F1 interspecific FO hybrid and obtained seedlings from backcrosses that will be beneficial for future breeding programs.
References
Abu-Qaoud H, Skirvin RM, Chevreau E (1990) In vitro separation of chimeral pears into their component genotypes. Euphytica 48:189–196
Anderson NO, Younis A, Optiz E (2009) Development of colored, non-vernalization -requiring seed propagated lilies. Acta Hortic 836:193–198
Anssour S, Krügel T, Sharbel TF, Saluz HP, Bonaventure G, Baldwin IT (2009) Phenotypic, genetic and genomic consequences of natural and synthetic polyploidization of Nicotiana attenuata and Nicotiana obtusifolia. Ann Bot 103:1207–1217
Bakhshaie M, Babalar M, Mirmasoumi M, Khalighi A (2010) Somatic embryogenesis and plant regeneration of Lilium ledebourii (Baker) Boiss., an endangered species. Plant Cell Tissue Organ Cult 102:229–235
Bakhshaie M, Khosravi S, Azadi P, Bagheri H, Van Tuyl JM (2016) Biotechnological advances in Lilium. Plant Cell Rep 35:1799–1826
Barba-Gonzalez R, Ramanna MS, Visser RGF, Van Tuyl JM (2005) Intergenomic recombination in F1 lily hybrids (Lilium) and its significance for genetic variation in the BC1 progenies as revealed by GISH and FISH. Genome 48:884–894
Barba-Gonzalez R, Lim KB, Van Tuyl JM (2014) Molecular cytogenetics in Lilium breeding. Acta Hortic 1027:129–142
Bi WL, Chen L, Guo L, Pan C, Yin ZF ,Wang QC (2015) Plant regeneration via embryo-like structures: histological observations and genetic stability in regenerants of Lilium spp. J Hortic Sci Biotech 90:626–634
Chandanie MA, Singh SK, Sindhu SS, Singh A, Tomar SMS, Prasad KV (2011) Efficacy of oryzalin as a potent chemical for in vitro induction of polyploids in Asiatic lily (Lilium hybrida L.) var. Polyanna. India J Genet 71:262–268
Dhooghe E, Denis S, Eeckhaut T, Reheul D, Van Labeke MC (2009) In vitro induction of tetraploids in ornamental Ranunculus. Euphytica 168:33–40
Dhooghe E, Van Laere K, Eeckhaut T, Leus L, Van Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult 104:359–373
Doležel J, Greilhuber J, Suda J (2007) Estimation of nuclear DNA content in plants using flow cytometry. Nat Protoc 2:2233–2244
Gomes SSL, Saldanha CW, Neves CS, Trevizani M, Raposo NRB, Notini MM, Santos MDO, Campos JMS, Otoni WC, Viccini LF (2014) Karyotype, genome size, and in vitro chromosome doubling of Pfaffia glomerata (Spreng.) Pedersen. Plant Cell Tissue Organ Cult 118:45–56
Han DS, Niimi Y (2008) Fertility restoring of interspecific hybrids between Lilium nobilissimum and L. regale by chromosome doubling. Acta Hortic 766:421–426
Hu FR (2007) The wild and artificial germplasm assessment by cytogenetics and mass propagation system establishment in Lilium. PhD thesis, Nanjing Forestry University, Beijing
Lim KB, Van Tuyl JM (2002) Identification of parental chromosomes and detection of ribosomal DNA sequences in interspecific hybrids of Lilium revealed by multicolor in situ hybridization. Acta Hortic 570:403–408
Lim KB, Chung JD, Van Kronenburg BCE, Ramanna MS, Hans de Jong J, Van Tuyl JM (2000) Introgression of Lilium rubellum Baker chromosomes into L. longiflorum Thunb.: a genome painting study of the F1 hybrid, BC1 and BC2 progenies. Chromosome Res 8:119–125
Lim KB, Wennekes J, Jong JHd, Jacobsen E, Van Tuyl JM (2001) Karyotype analysis of Lilium longiflorum and Lilium rubellum by chromosome banding and fluorescence in situ hybridisation. Genome 44:911–918
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Ning Y, Zhou H, Wang F (2003) The bulblet morphogenesis of Lilium × formolongi in scale propagation. Acta Hortic 30:229–231
Okazaki K (1996) Lilium species native to Japan, and breeding and production of Lilium in Japan. Acta Hortic 414:81–92
Omran A, Mohammad BN (2008) Polyploidization effect in two diploid cotton (Gossypium herbaceum L. and G. arboreum L.) species by colchicine treatments. African. J Biotechnol 7:102–108
Regalado JJ, Carmona-Martín E, Castro P, Moreno R, Gil J, Encina CL (2015) Study of the somaclonal variation produced by different methods of polyploidization in Asparagus officinalis L. Plant Cell Tissue Organ Cult 122:31–44
Rhee HK, Kim KS (2008) Interspecific hybridization and polyploidization in lily breeding. Acta Hortic 766:441–445
Rose JB, Kubba J, Tobutt KR (2000) Chromosome doubling in sterile Syringa vulgaris × S. pinnatifolia hybrids by in vitro culture of nodal explants. Plant Cell Tiss Organ Cult 63:127–132
Roy AT, Leggett G, Koutoulis A (2001) In vitro tetraploid induction and generation of tetraploids from mixoploids in hop (Humulus lupulus L.). Plant Cell Rep 20:489–495
Schwarzacher T, Heslop-Harrison JS (2000) Reprobing of preparations. In: Kingston F (ed) Practical in situ hybridization, BIOS Scientific Publishers Limited. Oxford, UK, p 110
Takamura T, Lim KB, Van Tuyl JM (2002) Effect of a new compound on the mitotic polyploidization of Lilium longiflorum and Oriental hybrid lilies. Acta Hortic 572:37–40
Takayama S, Misawa M (1980) Differentiation in Lilium bulbscales grown in vitro. Effects of activated charcoal, physiological age of bulbs and sucrose concentration on differentiation and scale leaf formation in vitro. Physiol Plant 48:121–125
Van Tuyl JM, Lim KB (2003) Interspecific hybridisation and polyploidisation as Tools in ornamental plant breeding. Acta Hortic 612:13–22
Van Tuyl JM, Van Holsteijn HCM (1996) Lily breeding research in the Netherlands. Acta Hortic 414:35–45
Van Tuyl JM, Van-Holsteijn HMC, Kwakkenbos AAM (1990) Research on polyploidy in interspecific hybridization of lily. Acta Hortic 266:323–330
Van Tuyl JM, Meijer B, Van Diën MP (1992) The use of oryzalin as an alternative for colchicine in in vitro chromosome doubling of Lilium and Nerine. Acta Hortic 325:625–630
Van Tuyl JM, Van Dijken A, Chi HS, Lim KB, Villemoes S, Van Kronenburg BCE (2000) Breakthroughs in interspecific hybridization of lily. Acta Hortic 508:83–90
Xu CP, Huang Z, Liao T, Li Y, Kang XY (2016) In vitro tetraploid plants regeneration from leaf explants of multiple genotypes in Populus. Plant Cell Tissue Organ Cult 125:1–9
Yan HJ, Xiong Y, Zhang HY, He ML (2016) In vitro induction and morphological characteristics of octoploid plants in Pogostemon cablin. Breeding Sci 66:169–174
Yin ZF, Zhao B, Bi WL, Chen L, Wang QC (2013) Direct shoot regeneration from basal leaf segments of Lilium and assessment of genetic stability in regenerants by ISSR and AFLP markers. In Vitro Cell Dev Biol- Plant 49:333–342
Zhang J, Zhang M, Deng XX (2007) Obtaining autotetraploids in vitro at a high frequency in Citrus sinensis. Plant Cell Tissue Organ Cult 89:211–216
Zhou SJ (2007) Intergenomic recombination and introgression breeding in Longiflorum × Asiatic lilies. Ph D thesis, Wageningen University, The Netherlands
Acknowledgements
The work was supported by grants from the National Forestry Public Welfare Industry Research Project (201204609) and the National Natural Science Foundation of China (31470106).
Author contributions
The authors have made the following declarations regarding their contributions: GXJ, XQZ conceived and designed the experiments; XQZ, QZC performed the experiments; and XQZ analyzed the data and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zhang, X., Cao, Q. & Jia, G. A protocol for fertility restoration of F1 hybrid derived from Lilium × formolongi ‘Raizan 3’ × Oriental hybrid ‘Sorbonne’. Plant Cell Tiss Organ Cult 129, 375–386 (2017). https://doi.org/10.1007/s11240-017-1184-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1184-9