Abstract
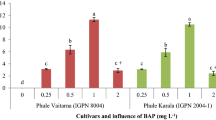
Effect of plant growth regulators and spermidine on somatic embryogenesis and regeneration was investigated in finger millet. Mature embryos, and 3 days old seedling-derived shoot apical meristems were cultured on Murashige and Skoog (MS) medium containing picloram, 2,4,5-trichlorophenoxyacetic acid, 2,4-dichlorophenoxyacetic acid (2,4-D) and 1-naphthaleneacetic acid. Improved embryogenesis (84.7 %) was found on MS medium containing 4.0 mg L−1 2,4-D and 0.5 mg L−1 kinetin in both explants. MS medium containing 1.5 mM spermidine along with 4.0 mg L−1 2,4-D and 0.5 mg L−1 kinetin produced highest frequency (90.4 %) of somatic embryogenesis from mature embryo derived callus in genotype ‘CO(Ra)-14’. On same medium somatic embryogenesis frequencies of ‘GPU-25’, ‘Try-1’ and ‘Piyur-2’ genotypes were 55.5, 85.3 and 58.7 %, respectively after 4 weeks of incubation in dark. MS medium containing 4.0 mg L−1 6-benzylaminopurine, 0.2 mg L−1 2,4-D and 1.5 mM spermidine was found to be optimum for shoot regeneration or somatic embryos in all four genotypes of finger millet. We also studied the influence of exogenous spermidine (2.0–4.0 mM) on regeneration of ‘CO(Ra)-14’ mature embryo-derived new and long-term (20–180 days old) calluses. Highest regeneration frequency 93.1 % and mean number of shoots 25.5 were produced on MS medium containing 3.0 mM spermidine, 4.0 mg L−1 BAP and 0.2 mg L−1 2,4-D using 60 days old callus. Regenerated shoots effectively rooted on half-strength MS medium and successfully acclimatized in soil with 100 % survival rate and they grew normally without showing any morphological variation. Genetic variation of in vitro derived plants and control plants were analyzed by RAPD markers.





Similar content being viewed by others
References
Ahmadabadi A, Ruf S, Bock R (2007) A leaf based regeneration and transformation system for maize (Zea mays L.). Transgenic Res 16:437–448
Alvarez I, Tomaro ML, Benavides MP (2003) Changes in polyamines, proline and ethylene in sunflower calluses treated with NaCl. Plant Cell Tissue Organ Cult 74:51–59
Bagni N, Mengoli M (1985) Characterization of a carrot callus line resistant to high concentrations of putrescine. Plant Growth Regul 3:371–380
Bajaj S, Rajam MV (1995) Efficient plant regeneration from long term callus cultures of rice by spermidine. Plant Cell Rep 14:717–720
Bajaj S, Rajam MW (1996) Polyamine accumulation and near loss of morphogenesis in long term callus cultures of rice. Restoration of plant regeneration by manipulation of cellular polyamine levels. Plant Physiol 112:1343–1348
Baskaran P, Rajeswari BR, Jayabalan N (2006) Development of an in vitro regeneration system in sorghum [Sorghum bicolor (L.) moench] using root transverse thin cell layers (tTCLs). Turk J Bot 30:1–9
Boget N, Torne JM, Willadino L, Santos M (1995) Variations in endogenous polyamine content of maize calli obtained from zygotic and androgenetic embryos. Plant Cell Tissue Organ Cult 40:139–144
Ceasar SA, Ignacimuthu S (2008) Efficient somatic embryogenesis and plant regeneration from shoot apex explants of different Indian genotypes of finger millet (Eleusine coracana (L.) Gaertn.). In Vitro Cell Dev Biol Plant 44:427–435
Ceasar SA, Ignacimuthu S (2010) Effects of cytokinins, carbohydrates and amino acids on induction and maturation of somatic embryos in kodo millet (Paspalum scorbiculatum Linn.). Plant Cell Tissue Organ Cult 102:153–162
Ceasar SA, Ignacimuthu S (2011) Agrobacterium-mediated transformation of finger millet (Eleusine coracana (L.) Gaertn.) using shoot apex explants. Plant Cell Rep 30:1759–1770
Chandra N, Kothari SL (1995) Advances in tissue culture and genetic transformation of cereals. J Indian Bot Soc 74:323–342
Chernobrovkina MA, Karavaev CV, Kharchenko PN, Melik-Sarkisov OS (2004) Somatic embryogenesis and morphogenetic potential of spring barley (Hordeum vulgare L.) in the system of technological genetic transformation. Biol Bull 31:332–336
Chong-Perez B, Reyes M, Rojas L, Ocana B, Perez B, Kosky RG, Angenon G (2012) Establishment of embryogenic cell suspension cultures and Agrobacterium-mediated transformation in banana cv. ‘Dwarf Cavendish’ (Musa AAA): effect of spermidine on transformation efficiency. Plant Cell Tissue Organ Cult 111:79–90
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Erland LAE, Mahmoud SS (2014) An efficient method for regeneration of lavandin (Lavandula x intermedia cv. ‘Grosso’). In Vitro Cell Dev Biol Plant 50:646–654
Fatima Z, Mujib A, Fatima S, Arshi A, Umar S (2009) Callus induction, biomass growth, and plant regeneration in Digitalis lanata Ehrh.: influence of plant growth regulators and carbohydrates. Turk J Bot 33:393–405
Feher A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult 74:201–228
Feirer RP, Mignon G, Litvay JD (1984) Arginine decarboxylase and polyamines required for embryogenesis in the wild carrot. Science 223:1433–1435
Feirer RP, Wann SR, Einspahr DW (1985) The effects of spermidine synthesis inhibitors on in vitro plant development. Plant Growth Regul 3:319–327
Filippov M, Miroshnichenko D, Vernikovskaya D, Dolgov S (2006) The effect of auxins, time exposure to auxin and genotypes on somatic embryogenesis from mature embryos of wheat. Plant Cell Tissue Organ Cult 84:213–222
Gill SS, Tuteja N (2010) Polyamines and abiotic stress tolerance in plants. Plant Signal Behav 5:26–33
Hema R, Vemanna RS, Sreeramulu S, Reddy CP, Senthil-Kumar M, Udayakumar M (2014) Stable expression of mtlD gene imparts multiple stress tolerance in finger millet. PLoS One 9(6):e99110
Hussain SS, Ali M, Ahmad M, Siddique KHM (2011) Polyamines: natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol Adv 29:300–311
Itoh JI, Sato Y, Nagato Y, Matsuoka M (2006) Formation, maintenance and function of the shoot apical meristem in rice. Plant Mol Biol 60:827–842
Kaur P, Kothari SL (2004) In vitro culture of kodo millet: influence of 2,4-D and picloram in combination with kinetin on callus initiation and regeneration. Plant Cell Tissue Organ Cult 77:73–79
Kothari SL, Agarwal K, Kumar S (2004) Inorganic nutrient manipulation for highly improved in vitro plant regeneration in finger millet—Eleusine coracana (L.) Gaertn. In Vitro Cell Dev Biol Plant 40:515–519
Kothari-Chajer A, Sharma M, Kachhwaha S, Kothari SL (2008) Micronutrient optimization results into highly improved in vitro plant regeneration in kodo (Paspalum scrobiculatum L.) and finger (Eleusine coracana (L.) Gaertn.) millets. Plant Cell Tissue Organ Cult 94:105–112
Kumar S, Agarwal K, Kothari SL (2001) In vitro induction and enlargement of apical domes and formation of multiple shoots in finger millet, Eleusine corcana (L.) Gaertn and crowfoot grass, Eleusine indica (L.) Garetn. Curr Sci 81:1482–1485
Kumar A, Mirza N, Charan T, Sharma N, Gaur VS (2014) Isolation, characterization and immunolocalization of a seed dominant CaM from finger millet (Eleusine coracana L. Gartn) for its studying functional role in differential accumulation of Ca in developing grains. Appl Biochem Biotechnol 172:2955–2973
Kusano T, Yamaguchi K, Berberich T, Takahashi Y (2007) Advances in polyamine research in 2007. J Plant Res 120:345–350
Latha AM, Rao KV, Reddy VD (2005) Production of transgenic plants resistant to leaf blast disease in finger millet (Eleusine coracana (L.) Gaertn). Plant Sci 169:657–667
Lee K, Hyesung J, Minkyun K (2002) Optimization of a mature embryo-based in vitro culture system for high-frequency somatic embryogenic callus induction and plant regeneration from japonica rice cultivars. Plant Cell Tissue Organ Cult 71:237–244
Mattoo AK, Minocha SC, Minocha R, Handa AK (2010) Polyamines and cellular metabolism in plants: transgenic approaches reveal different responses to diamine putrescine versus higher polyamines spermidine and spermine. Amino Acids 38:405–413
Monteiro M, Kevers C, Dommes J, Gaspar T (2002) A specific role for spermidine in the initiation phase of somatic embryogenesis in Panax ginseng CA Meyer. Plant Cell Tissue Organ Cult 68:225–232
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Palavan-Unsal N, Cotuk A, Kalac-Martini Y (2002) ∝-Difluoromethyl ornithine inhibits the growth of fungus Macrophomina phaseoli. Turk J Bot 26:43–45
Ramesh M, Murugaiah V, Gupta AK (2009) Efficient in vitro plant regeneration via leaf base segments of indica rice (Oryza sativa L.). Indian J Exp Biol 47:68–74
Rashid A (2002) Somatic embryogenesis from immature and mature embryos of a minor millet Paspalum scrobiculatum L. Plant Cell Tissue Organ Cult 69:71–77
Sanchez-Gras MC, Segura J (1988) Morphogenesis in vitro of Sideritis angustifolia: effects of auxins, benzyladenine and spermidine. Plant Sci 57:151–158
Santos MA, Camara T, Rodriguez P, Claparols I, Torne JM (1996) Influence of exogenous proline on embryogenic and organogenic maize callus subjected to salt stress. Plant Cell Tissue Organ Cult 47:59–65
Satish L, Ceasar SA, Shilpha J, Rency AS, Rathinapriya P, Ramesh M (2015) Direct plant regeneration from in vitro-derived shoot apical meristems of finger millet (Eleusine coracana (L.) Gaertn.). In Vitro Cell Dev Biol Plant 51:192–200
Shukla A, Lalit A, Sharma V, Vats S, Alam A (2015) Pearl and finger millets: the hope of food security. Appl Res J 1:59–66
Silva TER, Cidade LC, Alvim FC, Cascardo JCM, Costa MGC (2009) Studies on genetic transformation of Theobroma cacao L.: evaluation of different polyamines and antibiotics on somatic embryogenesis and the efficiency of uidA gene transfer by Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult 99:287–298
Slocum RD, Kaur-Sawhney R, Galston AW (1984) The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys 235:283–303
Sudhakar D, Duc LT, Bong BB, Tinjuangjun P, Maqbool SB, Valdez M, Jefferson R, Christou P (1998) An efficient rice transformation system utilizing mature seed-derived explants and a portable, inexpensive particle bombardment device. Transgenic Res 7:289–294
Sun L, Wu Y, Zou H, Su S, Li S, Shan X, Xi J, Yuan Y (2013) Comparative proteomic analysis of the H99 inbred maize (Zea mays L.) line in embryogenic and non-embryogenic callus during somatic embryogenesis. Plant Cell Tissue Organ Cult 113:103–119
Tiburcio AF, Kaur-Sawhney R, Galston AW (1987) Effect of polyamine biosynthetic inhibitors on alkaloids and organogenesis in tobacco callus cultures. Plant Cell Tissue Organ Cult 9:111–120
Tiburcio AF, Figueras X, Claparols I, Santos M, Torne JM (1991) Improved plant regeneration in maize callus cultures after pretreatment with DL-alpha-difluoromethyl arginine. Plant Cell Tissue Organ Cult 27:27–32
Venkatachalam L, Bhagyalakshmi N (2008) Spermine-induced morphogenesis and effect of partial immersion system on the shoot cultures of banana. Appl Biochem Biotechnol 151:502–511
Zhang Z, Honda C, Kita M, Nakayama CHM, Moriguchi T (2003) Structure and expression of spermidine synthase genes in apple: two cDNAs are spatially and developmentally regulated through alternative splicing. Mol Genet Genom 268:799–807
Acknowledgments
The author L. Satish sincerely thanks the University Grants Commission, New Delhi, India for financial support in the form of UGC Basic Scientific Research Fellowship. We thank Department of Small Millets, Millet Research Station, Tamil Nadu Agricultural University for providing seed material used in the present study. Also the authors gratefully acknowledge the Bioinformatics Infrastructure Facility of Alagappa University (funded by Department of Biotechnology, Government of India: Grant No. BT/BI/25/001/2006) for providing the computational facility.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Satish, L., Rency, A.S., Rathinapriya, P. et al. Influence of plant growth regulators and spermidine on somatic embryogenesis and plant regeneration in four Indian genotypes of finger millet (Eleusine coracana (L.) Gaertn). Plant Cell Tiss Organ Cult 124, 15–31 (2016). https://doi.org/10.1007/s11240-015-0870-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0870-8