Abstract
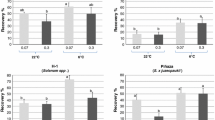
The clonal wild-species Malus collection at the Julius Kühn-Institute, Institute for Breeding Research on Fruit Crops, Germany, comprises 507 accessions of 46 species and represents one of the largest collections in Europe. Cryopreservation was applied to 77 accessions of 31 species and hybrids in the Malus collection using a winter vegetative-bud method. No significant differences were observed in the recovery of the two controls, untreated buds and desiccated buds, however, cryopreservation significantly decreased bud recovery, independent of the rootstock used for chip budding. Data from this investigation showed an effect on recovery of year and genotype between and within species. Of the 77 accessions, 62 had living buds, and recovery was >40 % for 37 and these averaged 68.5 % recovery. The initial moisture content, dehydration time, and morphology of the buds had no significant influence on recovery. The higher percentage of recovery in one experimental year was possibly the result of secondary buds that grew despite lethal injury to the primary meristems. Adaptations to the general protocol and the handling details were successful, and the cryopreservation of dormant buds will now be part of the conservation strategy for Malus genetic resources in Germany.



Similar content being viewed by others
References
Ashworth EN, Wisniewski ME (1991) Response of fruit tree tissue to freezing temperatures. HortScience 26:501–504
Berthaud J (1997) Strategies for conservation of genetic resources in relation with their utilization. Euphytica 96:1–12
Dusset S, Engelmann F, Noirot M (2003) Development of probabilistic tools to assit in the establishment and management of cryopreserved plant germplasm collections. CryoLetters 24:149–160
Engelmann F (2000) Importance of cryopreservation for the conservation of plant genetic Resources. In: Engelmann F, Takagi H (eds) Cryopreservation of tropical germplasm. Current research progress and application. Japan International Research Center for Agricultural Sciences/International Plant Genetic Resources Institute, Rome
Feng C-H, Cui Z-H, Li B-Q, Chen L, Ma Y-L, Zhao Y-H, Wang Q-C (2013) Duration of sucrose preculture is critical for shoot regrowth of in vitro-grown apple shoot-tips cryopreserved by encapsulation-dehydration. Plant Cell Tissue Org Cult 112:369–378
Flachowsky H, Höfer M (2010) The german fruit genebank, a decentral network for sustainable preservation of fruit genetic resources. J für Kulturpflanzen 62:9–16
Forsline PL, Towill LE, Waddell JW, Wiesner L (1995) Development of a base collection of Malus germplasm with cryopreserved buds. HortScience 30:872
Forsline PL, McFerson JR, Lamboy WF (1996) Optimizing bud harvest time and duration of temporary cold storage for cryopreservation of dormant apple buds. HortScience 31:646
Forsline PL, Towill LE, Waddell JW, Stushnoff C, Lamboy WF, McFerson JR (1998a) Recovery and longevity of cryopreserved dormant apple buds. J Am Soc Hortic Sci 123:365–370
Forsline PL, McFerson JR, Towill LE (1998b) Development of base and active collections of Malus germplasm with cryopreserved dormant buds. Acta Hort 484:75–78
Forsline PL, Aldwinckle HS, Dickson EE, Luby JJ, Hokaanson SC (2003) Collection, maintenance, characterization, and utilization of wild apples of Central Asia. In: Janick J (ed) Horticultural reviews, vol 29., Wild apple and fruit trees of central AsiaWiley, London, pp 1–62
Grout B, Vogiatzi C, Wetten A (2011) Rapid thawing (>5°C/min) of cryopreserved dormant buds of Malus can significantly reduce recovery of grafted explants. CryoLetters 32:542
Halmagyi A, Deliu C, Isach V (2010) Cryopreservation of Malus cultivars: comparison of two droplet protocols. Sci Hortic 124:387–392
Hao Y-J, Deng X-X (2003) Genetically stable regeneration of apple plants from slow growth. Plant Cell Tissue Org Cult 72:253–260
Höfer M (2007) Preliminary results of cryopreservation of Malus germplasm from the gene bank collection of the Institute of Fruit Breeding Dresden. Adv Hortic Sci 21:251–254
Höfer M, Keller ERJ (2013) Cryopreservation of Malus germplasm from the gene bank collection in Dresden, Germany.In: Abstract 2nd International symposium on plant cryopreservation Fort Collins, USA, 11–14 August 2013, p 91
Höfer M, Ali M, Sellmann J, Peil A (2014) Phenotypic evaluation and characterization of a collection of Malus species. Genet Resour Crop Evol 61:943–964
Janisiewicz WJ, Saftner RA, Conway WS, Forsline PhL (2008) Preliminary evaluation of apple germplasm from Kazakhstan for resistance to postharvest blue mold in fruit caused by penicillium expansum. HortScience 43:420–426
Jenderek M, Ambruzs B, Postman J, Ellis D (2010) Dormant bud cryopreservation for germplasm conservation. In Vitro Cell Dev Biol Anim 46:S–160
Jenderek MM, Forsline P, Postman J, Stover E, Ellis D (2011) Effect of geographical location, year, and cultivar on survival of Malus sp. dormant buds stored in vapors of liquid nitrogen. HortScience 46:1230–1234
Kovalchuk I, Lyudvikova Volgina M, Reed BM (2009) Medium, container and genotype all influence in vitro cold storage of apple germplasm. Plant Cell Tissue Org Cult 96:127–136
Kovalchuk I, Turdiev T, Mukhitdinova Z, Frolov S, Reed BM, Koirova G (2014) New techniques for rapid cryopreservation of dormant vegetative buds. Acta Hortic 1039:137–146
Kumar S, Volz RK, Alspach PA, Bus VGM (2009) Development of a recurrent apple breeding programme in New Zealand: a synthesis of results, and a proposed revised breeding strategy. Euphytica. doi:10.1007/s10681-009-0090-6
Kuo CC, Lineberger RD (1985) Survival of in vitro cultured tissue of ‘Jonathan’ apples exposed to −196°C. HortScience 20:764–767
Lambardi M, Rocncasaglia R, Previati A, De Carlo A, Dradi G, Da Re F (2006) In vitro slow growth storage of fruit rootstocks inside gas-tight or gas-permeable containers. Acta Hortic 725:483–488
Lambardi M, Benelli C, De Carlo D, Previati A (2009) Advances in the cryopreservation of fruit plant germplasm at the CNR-IVALSA Institute of Florence. Acta Hortic 839:237–244
Niino T, Sakai A, Yakuwa H, Nojiri K (1992) Cryopreservation of in vitro-grown shoot tips of apple and pear by vitrification. Plant Cell Tissue Org Cult 28:261–266
Peil A, Richter K, Höfer M, Hanke V (2004) Beschreibung des Feuerbrandbefalls am Institut für Obstzüchtung in Dresden-Pillnitz im Jahr 2003. Erwerbs-Obstbau 46:141–148
Reed BM (2001) Implementing cryogenic storage of clonally propagated plants. CryoLetters 22:97–104
Reed BM (2002) Implementing cryopreservation for long-term germplasm preservation in vegetatively propagated species. In: Towill LE, Bajaj YPS (eds) Biotechnology in agriculture and forestry, vol 50., Cryopreservation of plant germplasm IISpringer-Verlag, Berlin
Reed BM (2008) In: Reed BM (ed) Plant cryopreservation: A practical guide. Springer, New York, pp 3–13
Rehder A (1949) Bibliography of cultivated trees and shrubs. The Arnold Arboretum of Harvard University, Jamaica Plain
Sakai A (1960) Survival of the twig of woody plants at −196°C. Nature 4710:393–394
Sakai A (1966) Studies of frost hardiness in woody plants II. Effect of temperature on hardening. Plant Physiol 41:353–359
Sakai A, Nishiyama Y (1978) Cryopreservation of winter vegetative buds of hardy fruit trees in liquid nitrogen. HortScience 13:225–227
Stushnoff C (1987) Cryopreservation of apple genetic resources. Can J Plant Sci 67:1151–1154
Stushnoff C (1991) Cryopreservation of fruit crop genetic resources—implication for maintenance and diversity during conservation. HortScience 26:518–522
Stushnoff C, Seufferheld M (1995) Cryopreservation of apple (Malus species) genetic resources. In: Bajaj YPS (ed) Biotechnology in agriculture and forstry, vol 32., Cryopreservation of plant germplasm ISpringer Verlag, Berlin, pp 87–101
Toldam-Andersen TB, Nygaard TB, Krogholm KS (2007) Cryopreservation of dormant buds of apple cultivars in a mild maritime winter climate. Adv Hortic Sci 21:193–197
Towill LE, Bonnart R (2005) Cryopreservation of apple using non-desiccated sections from winter-collected scions. CryoLetters 26:323–332
Towill LE, Ellis DD (2008) Cryopreservation of dormant buds. In: Reed BM (ed) Plant cryopreservation—a practical guide. Springer, Science + Buisness Media, LLC, pp 421–426
Towill LE, Forsline PL (1999) Cryopreservation of sour cherry (Prunus cerasus L.) using a dormant vegetative bud method. CryoLetters 20:215–222
Towill LE, Forsline PL, Walters C, Waddell JW, Laufmann MJ (2004) Cryopreservation of Malus germplasm using a winter vegetative bud method: results from 1915 accessions. CryoLetters 25:323–334
Tyler NJ, Stushnoff C (1988a) The effects of prefreezing and controlled dehydration on cryopreservation of dormant vegetative apple buds. Can J Plant Sci 68:1163–1167
Tyler NJ, Stushnoff C (1988b) Dehydration of dormant apple buds at different stages of cold acclimation to induce cryopreservability in different cultivars. Can J Plant Sci 68:1169–1176
Vogiatzi C, Grout BWW, Wetten A, Toldam-Andersen TB (2010) Critical steps in cryopreservation of dormant winter buds collected under relatively mild winter conditions. Abstr Cryobiol 61:368
Vogiatzi C, Grout BWW, Wetten A, Toldam-Andersen TB (2011a) Cryopreservation of winter-dormant apple buds: I. variation in recovery with cultivar and winter conditions. CryoLetters 32:358–366
Vogiatzi C, Grout BWW, Wetten A, Toldam-Andersen TB (2011b) Cryopreservation of winter-dormant apple buds: II tissue water status after desiccation at −4°C and before further cooling. CryoLetters 32:367–376
Vogiatzi C, Grout BWW, Toldam-Andersen TB, Wulfsohn D (2011c) Dormant bud cryopreservation: secondary buds can affect the estimation of post-thaw survival. Acta Hortic 918:147–152
Vogiatzi C, Grout BWW, Wetten A (2012) Cryopreservation of winter-dormant apple: III—bud water status and survival after cooling −30°C and during recovery from cryopreservation. CryoLetters 33:160–168
Volk GM, Waddell J, Bonnart R, Towill L, Ellis D, Luffmann M (2008) High viability of dormant Malus buds after 10 years of storage in liquid nitrogen vapor. CryoLetters 29:89–94
Wu Y, Engelmann F, Zhao Y, Zhou M, Chen S (1999) Cryopreservation of apple shoot tip: importance of cryopreservation technique and of conditioning of donor plants. Cryo-Letters 20:121–130
Zhang Q, Li J, Zhao Y, Korban SS, Han Y (2012) Evaluation of genetic diversity in Chinese wild apple species along with apple cultivars using SSR markers. Plant Mol Biol Rep 30:539–546
Acknowledgments
I thank Simone Schöber for the excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Höfer, M. Cryopreservation of winter-dormant apple buds: establishment of a duplicate collection of Malus germplasm. Plant Cell Tiss Organ Cult 121, 647–656 (2015). https://doi.org/10.1007/s11240-015-0735-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0735-1