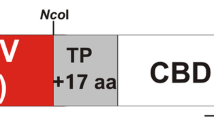
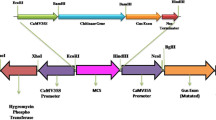
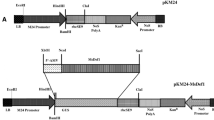
Abstract
Mammalian infectious diseases are widespread, and some are becoming difficult to control due to inappropriate use of antibiotics. This has contributed to incidence of bacterial strains with resistance to commonly used antibiotics. Thus, effective alternative antibiotics are essential for treatment of infectious diseases. Antimicrobial peptides are viable alternatives to address this problem. Among those, protegrin-1 (PG-1) is a broad-spectrum antimicrobial peptide. In this study, a magnICON was used to express the PG-1 peptide in Nicotiana tabacum, using a transient expression system mediated by Agrobacterium tumefaciens transfection. Reverse-transcriptase polymerase chain reaction (RT-PCR) and Northern blot analyses of transformed N. tabacum were employed to detect viral replicons, 290 bp and 6.1 kb. SDS/PAGE revealed presence of a band corresponding to the molecular weight of PG-1 (2.1 kDa), which was absent in wild-type N. tabacum. Antimicrobial/antifungal assays of protein extracts from transiently transformed N. tabacum were performed, and these demonstrated that PG-1 peptide activity in these plant tissues was viable and contributed to inhibition of 53.2 % of Klebsiella pneumoniae, 70.2 %, of Staphylococcus aureus, 56.6 % of Escherichia coli, 72 % of Mycobacterium bovis BCG, and 70 % of Candida albicans cultures. No inhibition of any of these fungal and bacterial pathogens was detected when wild-type N. tabacum extracts were used. Therefore, PG-1 produced in plant cells of infiltrated tobacco was active and controlled growth of several bacterial and fungal human pathogens.



Similar content being viewed by others
Abbreviations
- AMPs:
-
Antimicrobial peptides
- TMV:
-
Tobacco mosaic virus
- GFP:
-
Green fluorescent protein
- PG1:
-
Protegrin-1
- PCR:
-
Polymerase chain reaction
- RT-PCR:
-
Reverse-transcriptase polymerase chain reaction
- TSP:
-
Total soluble protein
- Rs-ASP2:
-
Signal peptide of the Raphanus antifungal protein 2
- CFU:
-
Colony-forming unit
References
Alvarez ML, Cardineau GA (2010) Prevention of bubonic and pneumonic plague using plant-derived vaccines. Biotechnol Adv 28:184–196
Anthony KB, Fishman NO, Linkin DR, Gasink LB, Edelstein PH, Lautenbach E (2008) Clinical and microbiological outcomes of serious infections with multidrug-resistant gram-negative organisms treated with tigecycline. Clin Infect Dis 46:567–570
Barlow PG, Svoboda P, Mackellar A, Nash AA, York IA, Pohl J, Davidson DJ, Donis RO (2011) Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS ONE 6:e25333
Bradford MM (1968) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen J, Falla TJ, Liu H, Hurst MA, Fujii CA, Mosca DA, Embree JR, Loury DJ, Radel PA, Chang C, Gu L, Fiddes JC (2000) Development of protegrins for the treatment and prevention of oral mucositis: structure-activity relationships of synthetic protegrin analogues. Biopolymers 55(1):88–98
Cho Y, Turner JS, Dinh N, Lehrer R (1998) Activity of protegrins against yeast-phase Candida albicans. Infect Immun 66:2486–2493
Cole A (2005) Antimicrobial peptide microbicides targeting HIV. Protein Pept Lett 12:41–47
Colgrave ML, Kotze AC, Huang YH, O’Grady J, Simonsen SM, Craik DJ (2008) Cyclotides: natural, circular plant peptides that possess significant activity against gastro-intestinal nematode parasites of sheep. Biochemistry 47:5581–5589
DeLucca AJ, Bland JM, Jacks TJ, Grimm C, Cleveland TJ (1997) Walsh, fungicidal activity of cecropin A. Antimicrob Agents Chemother 41:481–483
Field D, Connor PM, Cotter PD, Hill C, Ross RP (2008) The generation of nisin variants with enhanced activity against specific gram positive pathogens. Mol Microbiol 69:218–230
Findlay B, Zhanel GG, Schweizer F (2010) Cationic amphiphiles, a new generation of antimicrobials inspired by the natural antimicrobial peptide scaffold. Antimicrob Agents Chemother 54:4049–4058
Gleba Y, Klimyuk V, Marillonnet S (2005) Magnifection-a new platform for expressing recombinant vaccines in plants. Vaccine 23:2042–2048
Gleba Y, Klimyuk V, Marillonnet S (2007) Viral vectors for the expression of proteins in plants. Curr Opin Biotechnol 18:134–141
Hulscher ME, Grol RP, van der Meer JW (2010) Antibiotic prescribing in hospitals: a social and behavioural scientific approach. Lancet Infect Dis 10:167–175
Ireland DC, Wang CK, Wilson JA, Gustafson KR, Craik DJ (2008) Cyclotides as natural anti-HIV agents. Biopolymers 90:51–60
Jenssen H, Hamill P, Hancock R (2006) Peptide antimicrobial agents. Clin Microbiol Rev 19(3):491–511
Langham AA, Ahmad AS, Kaznessis YN (2008) On the nature of antimicrobial activity: a model for protegrin-1 pores. J Am Chem Soc 130:4338–4346
Lee SB, Li B, Jin S, Daniell H (2011) Expression and characterization of antimicrobial peptides retrocyclin-101 and protegrin-1 in chloroplasts to control viral and bacterial infections. Plant Biotechnol J 9(1):100–115
Li Y, Geng Y, Song H, Zheng G, Huan L, Qiu B (2004) Expression of human lactoferrin N-lobe in Nicotiana benthamiana with potato virus X-based agroinfiltration. Biotechnol Lett 26:953–957
Marcos JF, Munñoz A, Pérez-Payá E, Misra S, López-García B (2008) Identification and rational design of novel anti- microbial peptides for plant protection. Annu Rev of Phytopathol 46:273–301
Marillonnet S, Giritch A, Gils M, Kandzia R, Klimyuk V, Gleba Y (2003) In planta engineering of viral RNA replicons. Efficient assembly by recombination of DNA modules delivered by Agrobacterium. PNAS 101:6852–6857
Matejuk A, Leng Q, Begum MD, Woodle MC, Scaria P, Chou ST, Mixson AJ (2010) Peptide-based antifungal therapies against emerging infections. Drugs Future 35(3):197
Menassa R, Du C, Yin ZQ, Ma S, Poussier P, Brandle J, Jevnikar AM (2007) Therapeutic effectiveness of orally administered transgenic low-alkaloid tobacco expressing human interleukin-10 in a mouse model of colitis. Plant Biotechnol J 5(1):50–59
Qu X, Harwig SL, Shafer W, Lehrer R (1997) Protegrin structure and activity against Neisseria gonorrhoeae. Infect Immun 65:636–639
Rosales-Mendoza S, Paz-Maldonado LMT, Govea-Alonso DO, Korban SS (2012) Engineering production of antihypertensive peptides in plants. Plant Cell Tiss Org Cult. doi:10.1007/s11240-012-0231-9
Rymerson RT, Babiuk LA, Menassa R, Vanderbeld B, Brandle JE (2003) Immunogenicity of the capsid protein VP2 from porcine parvovirus expressed in low alkaloid transgenic tobacco. Mol Breed 11:267–276
Saioth H, Kiba A, Nishihara M, Yamamura S, Suzuki K, Terauchi R (2000) Production of antimicrobial defensin in Nicotiana benthamiana with a potato virus X vector. MPMI 14:111–115
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York 78
Staub JM, Garcia B, Graves J, Hajdukiewicz P, Hunter P, Nehra N, Paradkar V, Schlittler M, Carroll J, Spatola L, Ward D, Ye G, Russell D (2000) High-yield production of a human therapeutic protein in tobacco chloroplasts. Nat Biotechnol 18:333–338
Steinberg DA, Hurst MA, Fuji CA, Kung AHC, Ho JF, Cheng FC, Loury DJ, Fiddes JC (1997) Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob Agents Chemother 41:1738–1742
Streatfield S (2007) Approaches to achieve high-level heterologous protein production in plants. Plant Biotechnol J 5:2–15
Terras FRG, Schoofs HME, De Bolle MFC, Leuvent FV, Rees SB, Vanderleyden J, Cammute BPA, Breoekaert WF (1992) Analysis of two novel classes of plant antifungal proteins from Radish (Raphanus sativus L.) seeds. J Biol Chem 267:15301–15309
Terras FRG, Eggermont K, Kovaleva V, Raikhel NV, Osborn RW, Kaster A, Rees SB, Torrekens S, Van Leuven F, Vanderleyden J, Cammue BPA, Broekaert WF (1995) Small cysteine-rich antifungal proteins from radish: their role in host defense. Plant Cell 7:573–588
Tremblay R, Wang D, Jevnikar AM, Shengwu M (2010) Tobacco, a highly efficient green bioreactor for production of therapeutic proteins. Biotechnol Adv 28:214–221
Von Haehling S, Morley JA, Anker SD (2010) An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle 1:129–133
WHO (2000). http://www.who.int/infectious-disease-report/2000/
Willey JM, van der Donk WA (2007) Lantibiotics: peptides of diverse structure and function. Annu Rev Microbiol 61:477–501
Yeman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55:27–55
Zaiou M (2007) Multifunctional antimicrobial peptides: therapeutic targets in several human diseases. J Mol Med 85:317–329
Acknowledgments
We would like to acknowledge Dr. Menassa who kindly donated the seeds producing low-levels of nicotine of N. tabacum cv. 81V9 and Icon Genetics for the pro-vectors gifted for this research. We also thank CONACyT Mexico for the grants 37048-B, 56980 and 154790 and scholarship 232052 granted to Patiño-Rodríguez for his PhD studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patiño-Rodríguez, O., Ortega-Berlanga, B., Llamas-González, Y.Y. et al. Transient expression and characterization of the antimicrobial peptide protegrin-1 in Nicotiana tabacum for control of bacterial and fungal mammalian pathogens. Plant Cell Tiss Organ Cult 115, 99–106 (2013). https://doi.org/10.1007/s11240-013-0344-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-013-0344-9