Abstract
Dengue (DEN) is one of the most important emerging mosquito-borne viral human diseases. Therefore, an effective dengue vaccine with immune responses against all four dengue virus serotypes is highly needed. A fusion gene encoding a synthetic consensus envelope protein domain III (scEDIII) of dengue virus with neutralizing activity against the four dengue virus serotypes and with the B subunit of cholera toxin (CTB) to increase its mucosal immunogenicity was constructed and was introduced into rice callus under the control of the inducible rice amylase 3D promoter expression system. The integration and expression of the CTB-scEDIII fusion gene in transgenic rice callus were confirmed by genomic DNA PCR amplification, Northern, and Western blot analyses, respectively. The biological binding activity of the CTB-cEDIII fusion protein to its GM1-ganglioside receptor was confirmed via GM1-ELISA with anti-CT and anti-dengue virus antibodies. Delivery of the CTB-cEDIII fusion protein into mucosal immune inductive sites (including M cells) in BALB/c mice was confirmed by in vitro and in vivo antigen uptake assays. These results showed that the CTB-cEDIII fusion protein was produced in the transgenic rice callus, and that plant-produced ligand fusion antigen proteins have the potential to be targeted to the mucosal immune system for improvement of the overall immune responses.
Similar content being viewed by others
Introduction
Dengue is one of the most important emerging mosquito-borne viral diseases in tropical and sub-tropical areas. Although mainly occurring in sub-tropical regions, this disease infects approximately 50–100 million individuals worldwide annually (Gubler 2002). Recently, it has become a significant public health problem, transcending geographical boundaries and placing nearly 50 % of the global population at risk (Siddiqui et al. 2009). Intensive studies to produce effective vaccines against dengue are needed because of the potential and considerable economic burden of this disease.
The dengue virus E protein is associated with the host cell receptor–binding motif of domain III (EDIII). In earlier studies (Fonseca et al. 1991), recombinant proteins based on EDIII were expressed in E. coli and yeast, and efficient immunogenicity was demonstrated (Batra et al. 2010; Block et al. 2010; Brandler et al. 2010; Guzman et al. 2010; Etemad et al. 2008). Numerous efforts to develop a vaccine for dengue have been initiated since the early 1940s, and several types of vaccines have been reported (Murrell et al. 2011). To date, an effective dengue vaccine has yet to be licensed for human use. Dengue virus consists of four antigenically different serotypes (DEN 1–4), which makes it difficult to develop an effective dengue vaccine as infection with just one of the dengue virus serotypes can lead to the full spectrum of dengue symptoms: fever, potentially life-threatening dengue hemorrhagic fever, or dengue shock syndrome after heterologous consecutive infections, which is referred to as antibody-dependent enhancement (ADE) (Huang et al. 2006). The tetravalent dengue vaccines are thought to provide protection against all serotypes without ADE (Swaminathan et al. 2010; Miller 2010). For these vaccines, the consensus amino acid sequence was deduced by alignment of EDIII sequences from different isolates of the four different dengue viral serotypes. Mice immunized with the recombinant consensus envelope domain III (cEDIII) developed neutralizing antibodies against all four serotypes of dengue virus (Chiang et al. 2011; Leng et al. 2009).
The production of commercial and pharmaceutical proteins in transgenic plants has been actively researched, resulting in a fast and flexible production system by advances in genetic engineering. Plant-based production systems offer safe and inexpensive vaccines with the capacity to deliver antigens to mucosal immune targets by oral vaccination (Kim et al. 2010b; Youm et al. 2010; Mason et al. 1996). However, the low production level of antigen proteins related to oral tolerance and low immune response is a barrier to expanded vaccine production using this process and should ideally be resolved before this method is used as a viable edible vaccine. To achieve high yields from plants, some recombinant proteins have significantly increased their stable expression through sub-cellular targeting (Pelham 1990; Yasuda et al. 2006) and chloroplast transformation, with levels of up to 47 % total soluble protein (TSP) having been reported (Chebolu and Daniell 2009). Genetic engineering approaches, like gene optimization, strong promoter use, efficient untranslated leader and 3′ untranslated sequences (UTRs) have also been employed for such purpose (Shin et al. 2003). In addition, transient expression systems have become a commonly used method for expressing high-value proteins (Gleba et al. 2005). Recombinant protein yields of up to 80 % TSP using transient expression systems have been reported (Marillonnet et al. 2004), and the rapid, high-yield production of individualized idiotype vaccines for non-Hodgkin’s lymphoma was successfully achieved in Nicotiana benthamiana (Bendandi et al. 2010).
Another strategy to increase the immune response in mucosal immune systems is the fusion of antigen proteins with ligands which have the ability to deliver the fused antigen protein to mucosal immune induction sites for enhanced antigen uptake into mucosal immune cells. Known representative ligands in transgenic plants are the cholera toxin B subunit (CTB) and enterotoxigenic E. coli enterotoxin B subunit (LTB). Heat labile toxin (LT), produced by E. coli, and cholera toxin (CT), produced by Vibrio cholera, are members of the AB5 toxin family. CT is composed of a non-toxic B subunit, which is responsible for cell attachment, and a toxicogenic A subunit, which is post-translationally cleaved into the A1 and A2 peptides (Sanchez and Holmgren 2008). The B subunit has a donut-shaped pentameric conformation and binds to GM1-ganglioside present on all nucleated mammalian cells; this receptor is also abundant in intestinal epithelial cells (Liljeqvist et al. 1997). CTB is not only an antigen protein against CT (Yuki et al. 2009), but also one of the major candidates for an oral delivery carrier system for other vaccine-relevant antigens through chemical or genetic coupling with various protein or peptide antigens (Boyhan and Daniell 2011; Kim et al. 2009b). A mucosal immunopotentiating effect revealed by eliciting high serum IgG antibody titers as well as secretory IgA in mucosal secretions has been reported in some studies (Boberg et al. 2008; Sanchez et al. 2004). CTB has been suggested as a stable carrier molecule and fusion partner for other antigenic peptides or proteins in order to achieve high levels of antigen expression in plants (Streatfield 2006). Concerns regarding retained functionality of the CTB- or LTB-fusion proteins have been inconclusive (Lipscombe et al. 1991; Schodel et al. 1990), and recent research efforts have shown functional activity of CTB or LTB fusion proteins (Huy et al. 2011; Kim et al. 2010c, 2011; Rosales-Mendoza et al. 2011; Shin et al. 2011).
In this study, the rice codon-optimized cEDIII gene, whose protein product allows for protection against all four serotypes, was fused to the CTB protein to develop a plant-based edible vaccine with the potential to elicit an increased mucosal immune response. The CTB-cEDIII fusion protein was expressed under the control of the rice amylase 3D (RAmy3D) promoter, which is a strong inducible promoter under sugar starvation conditions, within a rice cellular expression system. The biological functionality of CTB-cEDIII fusion proteins for binding to mucosal immune cells was investigated in a mouse model.
Materials and methods
Construction of plant expression vectors
A gene encoding the consensus dengue virus envelope protein domain III (103 aa, scEDIII), which was modified based on plant-optimized codon usage (Kim et al. 2012) and with cross-neutralizing activity against four dengue virus serotypes (Leng et al. 2009), was genetically fused to the CTB gene. The CTB gene was amplified from pMYV498, which contains the CTB-EDIII gene (Kim et al. 2010c), with gene-specific primers (forward primer CTBFBG: 5′-AGA TCT ACA CCT CAA AAT-3′ and reverse primer CTBRB: 5′-GC GGA TCC CGG GCC TGG GCC ATT-3′). The primers contain BglII and BamHI sites, respectively (underlined) for convenient subcloning. The PCR product was cloned into the pGEM-T Easy vector (Promega, Madison, WI), and its sequence was confirmed by DNA sequence analysis. The CTB gene was excised with BglII and BamHI and subcloned into the BamHI site of pMYV657 (Kim et al. 2012) to generate plasmid containing the CTB-scEDIII fusion gene (pMYV659). The CTB-scEDIII fusion gene containing a rice amylase 3D signal peptide was under the control of the promoter and the 3′ untranslated region of the rice amylase 3D gene. A flexible Gly-Pro-Gly-Pro (GPGP) linker peptide was located between the CTB and cEDIII proteins (Fig. 1a).
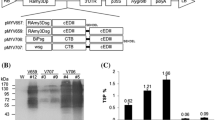
Transformation of rice calli with the CTB-cEDIII fusion gene. Construction of a plant expression plasmid containing the consensus domain III (scEDIII) of dengue virus glycoprotein fused with the cholera toxin B (CTB) subunit (pMYV659) under the control of a promoter (RAmy3D) and the 3′ untranslated region (3′UTR) of rice amylase 3D gene (a). The CTB-scEDIII fusion gene was detected by genomic DNA PCR amplification with CTB-scEDIII gene-specific primers in putative transgenic rice callus. Lane PC, plant expression plasmid containing the CTB-scEDIII fusion gene as the positive control; Lane M, 100 bp DNA ladder (ELPIS BIOTECH, Seoul, Korea); Lane NC, non-transgenic rice callus as the negative control; Lanes 1–12, putative transgenic rice callus (b). Total RNA extracts from transgenic rice callus after induction via sugar starvation were subjected to Northern blot analysis to detect CTB-scEDIII fusion gene transcripts. Lane NC, total RNA extracts of non-transgenic rice callus used as the negative control; Lanes 1–12, total RNA extracts of transgenic rice callus (c)
Rice callus transformation
Rice callus (Oryza sativa L. cv. Dongin) were prepared and transformed with pMYV659 via particle-bombardment-mediated transformation (Chen et al. 2002). After 3–5 days, the rice callus were transferred to N6 selection media supplemented with 2,4 dichlorophenoxyacetic acid (2 mg l−1), sucrose (30 g l−1), proline (0.5 g l−1), glutamine (0.5 g l−1), casein enzymatic hydrolysate (0.3 g l−1), gelite (2 g l−1), and hygromycin B (50 mg l−1) as the antibiotic for selection and allowed to grow for 2–3 weeks.
Genomic DNA PCR amplification
Genomic DNA in putative transgenic and non-transgenic rice callus was purified using a ZymoBead™ Genomic DNA Kit (Zymo Research, Orange, CA) and was used for genomic DNA PCR analysis. The presence of the CTB-scEDIII fusion gene in rice callus was determined using gene specific forward (CTBFBG) and reverse (5′-GGT ACC GGA GGA GCC CTT-3′) primers. The PCR mixture contained 100 ng of genomic DNA, 10 pmol primers, 200 μM dNTPs, 1 × Taq polymerase buffer (1 mM Tris–Cl, pH 8.8, 5 mM KCl, and 0.01 % Triton X-100), 1.5 mM MgCl2, and 2 U i-Taq polymerase (iNtRON Biotechnology, Seoul, Korea) in a total reaction volume of 20 μl. Plant expression vector pMYV659 DNA (50 ng) was used as the positive control. The amplified DNA was resolved using 1.0 % agarose gel electrophoresis.
Northern blot analysis
Total RNA was extracted from non-transgenic and transgenic rice callus tissue 5 days after induction under sugar starvation conditions using Trizol Reagent (Molecular Research Center, Inc., Cincinnati, OH) according to the supplier’s instructions. Thirty micrograms of total RNA was resolved on 1.2 % formaldehyde-containing agarose gels and then transferred to a Hybond-N+ membrane (Amersham–Pharmacia Biotech, Piscataway, NJ). The blot was hybridized overnight with a 32P-labeled random-primed (Promega) CTB-scEDIII probe at 65 °C in modified Church buffer (pH 7.4) that contained 1 mM EDTA, 250 mM Na2HPO4 · 7H2O, 1 % hydrolyzed casein, and 7 % SDS in a hybridization incubator (Finemould Precision Ind., Seoul, Korea). The blot was washed twice with 2 × SSC plus 0.1 % SDS and twice with 2 × SSC plus 1 % SDS for 15 min each at 65 °C. Hybridized bands were detected by autoradiography using X-ray film (Fuji Photo Film Co. HR-G30, Tokyo, Japan).
Western blot analysis
Non-transgenic and transgenic rice callus were analyzed to detect CTB-cEDIII fusion proteins by Western blot analysis. Transgenic rice callus were extracted 7 days after induction under sugar starvation conditions with extraction buffer (200 mM Tris–Cl, pH 8.0, 100 mM NaCl, 400 mM sucrose, 10 mM EDTA, 14 mM 2-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride, and 0.05 % Tween-20). Thirty micrograms of total soluble protein (TSP) as determined by Bradford protein assay (Bio-Rad, Inc., Hercules, USA) was separated by 15 % sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) at 120 V for 2-2.5 h after boiling for 5 min or no boiling in Tris–glycine buffer (25 mM Tris–Cl, 250 mM glycine, pH 8.3, and 0.1 % SDS). The separated protein bands were transferred from the gel to a Hybond C membrane (Amersham Pharmacia Biotech RPN303C) using a mini-transblot apparatus (Bio-Rad) at 150 mA for 2 h. Nonspecific antibody binding was blocked by 5 % non-fat dry milk in TBS buffer (20 mM Tris–Cl, pH 7.5, and 500 mM NaCl), followed by washing in TBS buffer for 5 min. The membrane was incubated for 2 h in a 1:5,000 dilution of rabbit anti-CT antibody (Sigma, St. Louis, MO) or a 1:2,500 dilution of mouse anti-dengue virus antibody (Serotech, Oxford, UK) in TBST antibody dilution buffer (TBS with 0.05 % Tween-20 and 2 % non-fat dry milk), followed by three washes in TBST buffer (TBS with 0.05 % Tween-20). The membrane was incubated for 2 h in a 1:5,000 dilution of goat anti-rabbit or mouse IgG conjugated with alkaline phosphatase (Sigma). The membrane was washed twice with TBST buffer and once with TMN buffer (100 mM Tris–Cl, pH 9.5, 5 mM MgCl2 and 100 mM NaCl). After washing, color was developed using premixed BCIP/NBT solution (Sigma).
Gm1-ELISA
Transgenic rice callus lines showing high expression levels of CTB-cEDIII fusion proteins by Western blot analysis were selected for suspension culture. The transgenic rice callus were harvested using a vacuum pump 7 days after induction under sugar starvation conditions. The biological activity of CTB-cEDIII fusion proteins in transgenic rice callus was detected by GM1-enzyme-linked immunosorbent assay (GM1-ELISA). The total soluble protein was extracted by grinding the lyophilized powder (100 mg dried cell weight) of transgenic and non-transgenic rice callus with a chilled mortar and pestle to a fine powder with gradual addition to extraction buffer (200 mM Tris–Cl, pH 8.0, 100 mM NaCl, 400 mM sucrose, 10 mM EDTA, 14 mM 2-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride, and 0.05 % Tween-20). For GM1-ELISA, a 96-well microtiter plate was coated with 100 μl monosialoganglioside GM1 (3.0 μg ml−1) per well (Sigma), covered with plastic wrap, and incubated at 4 °C overnight. The wells were washed three times with PBST buffer (PBS plus 0.05 % Tween-20), blocked by the addition of 300 μl 1 % BSA in PBS buffer per well, and incubated at 37 °C for 2 h, followed by 3 washes with PBST buffer. The total soluble protein was diluted 1:10 in PBS buffer. Serial dilutions (100 μl per well) of protein extracts and a commercial CTB (Sigma) (10 ng of protein) used as a standard were loaded and incubated at 37 °C for 2 h. The plates were incubated with a 1:5,000 dilution of rabbit anti-CT antibody or a 1:2,500 dilution of mouse anti-dengue virus antibody in 0.01 M PBS buffer containing 0.1 % BSA for 2 h at 37 °C and then washed three additional times with PBST buffer. The plates were incubated with a 1:5,000 dilution of goat anti-rabbit or mouse IgG conjugated with alkaline phosphatase in 0.1 % BSA and washed three times with PBST buffer. The plates were developed by the addition of 100 μl AP substrate for 12 min at room temperature in the dark. Absorbance was measured at 405 nm using a SpectraCountTM (Packard Instrument Co., Downers Grove, IL) ELISA reader. Biologically-active CTB-cEDIII fusion protein levels from transgenic rice dried callus were calculated by GM1-ELISA with anti-CT antibody.
In vitro and in vivo antigen uptake assays
A gut loop containing Peyer’s patches from male BALB/c mice (Charles River Technology, MA, through Orient Bio., Sungnam, Korea) that had been fasted overnight were used for in vitro CTB-cEDIII fusion antigen binding assays. The gut loops were washed with ice-cold PBS buffer and fixed with 4 % paraformaldehyde. The tissue was blocked with 2.5 % BSA, 0.1 % glycine, and anti-mouse CD16/32 antibody/PBS and protein extracts from non-transgenic and transgenic rice callus (50 μg CTB-cEDIII fusion protein) were applied for 2 h for binding. The tissue was then stained with Alexa Flour 350® conjugated-WGA (Invitrogen, Carlsbad, CA), rhodamine-labeled UEA-1 (Santa Cruz Biotechnology, Santa Cruz, CA), and mouse anti-dengue virus antibody followed by fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (BD Bioscience, Franklin Lakes, NJ). The tissues were then analyzed using confocal laser scanning microscopy (CLSM; LSM 510 META; Carl Zeiss, Thornwood, NY). Male BALB/c mice were orally administrated protein extracts from non-transgenic rice or transgenic rice callus containing CTB-cEDIII fusion protein for 10 min and then euthanized. Gut loops containing Peyer’s patches from the mice were prepared, washed with ice-cold PBS, and fixed with 4 % paraformaldehyde. After blocking with 2.5 % BSA, 0.1 % glycine, and anti-mouse CD16/32 antibody/PBS, the tissue was stained with Alexa Flour 350® conjugated-WGA, rhodamine-conjugated UEA-1, and anti-dengue virus antibody followed by FITC-conjugated anti-mouse IgG, and was then analyzed by CLSM.
Results
Construction of the plant expression vector and genomic DNA PCR analysis
A gene encoding the consensus EDIII (scEDIII) protein of dengue virus envelope glycoprotein was synthesized based on plant-optimized codon usage (Kim et al. 2012), which was deduced in accordance with the amino acid sequence containing cross-neutralizing activity against four dengue virus serotypes. The gene encoding the CTB was fused to the N-terminus of the scEDIII gene in order to overcome low immune response and immune tolerance due to low production levels of the target protein. The CTB-scEDIII fusion gene was inserted into the pMYV659 plant expression vector under the control of the RAmy3D promoter, which is a sucrose starvation-inducible promoter. Rice callus were transformed with the expression plasmid via particle bombardment-mediated transformation methods, and putative transgenic callus were selected under hygromycin pressure. Twelve independent, putative transgenic rice callus with the CTB-scEDIII fusion gene were selected 2–4 weeks after transformation. A DNA fragment corresponding in size to the CTB-scEDIII gene including the signal sequence (720 bp) was amplified by genomic DNA PCR amplification with primer sets specific to the CTB-scEDIII fusion gene in all 12 transgenic rice callus. DNA bands for CTB-scEDIII fusion genes were not detected in non-transgenic rice callus (Fig. 1b).
Expression of CTB-scEDIII fusion gene in transgenic rice callus
Transcription of the CTB-scEDIII fusion gene was confirmed in transgenic rice callus using a [32P]-labeled CTB-scEDIII fusion gene probe by Northern blot analysis. Positive signals for the CTB-scEDIII fusion gene were detected in the total RNA extracted from 11 of the 12 transgenic rice callus lines, and no signal was found in non-transgenic rice callus (Fig. 1c). Additional bands below the positive signal for the CTB-scEDIII fusion gene were also detected in 2 transgenic lines (lanes 2 and 8, Fig. 1c), which may be a truncated form of the CTB-scEDIII transcripts. Six transgenic rice callus lines showing a strong signal in Northern blot analysis were tested for production of the CTB-cEDIII fusion protein. Results of immunoblotting with anti-CT antibody showed the same pattern as that of the anti-dengue virus antibody, indicating that the CTB and cEDIII proteins were located at the same position and expressed as a single protein. The results of immunoblotting with both anti-CT and anti-dengue virus antibodies under non-boiled conditions showed two main bands: the higher band was about 130 kDa, and the lower band was about 95 kDa (Fig. 2a, b). Under boiled conditions, two bands were detected in the transgenic rice callus lines (Figs. 2c, d). No bands corresponding to the CTB-cEDIII fusion protein were detected in non-transgenic rice callus protein extracts.
Western blot analysis of the CTB-cEDIII fusion protein. The CTB-cEDIII fusion protein produced in transgenic rice callus after induction with sucrose starvation were separated via SDS-PAGE and subjected to Western blot analysis with anti-CT (a, c) or anti-dengue virus antibodies (b, d) either under boiling conditions for 5 min (c, d) or without boiling (a, b). Lane PC1, commercial bacterial CTB; Lane PC2, purified EDIII (serotype 2) in E. coli; Lane NC, non-transgenic rice callus protein extracts used as a negative control; Lanes 2, 3, 6, 7, 8, and 12, protein extracts from transgenic rice callus. Arrows indicate assembled CTB-cEDIII fusion proteins
Biological activity and quantification of the CTB-cEDIII fusion protein
Transgenic rice callus line #12 had the highest CTB-cEDIII fusion protein level based on Western blot analysis and was therefore selected for the measurement of biological activity and expression levels. Antigen immunogenicity can be improved when coupled to CTB as a carrier and an adjuvant due to both the increased uptake of coupled antigen across the mucosal barrier and the more efficient presentation of coupled antigens by intestinal mucosal immune cells. The binding of a CTB fusion protein to GM1-ganglioside is needed for internalization and activation of the fusion protein. The transgenic rice callus were lyophilized at 7 days after induction under sugar starvation conditions. The GM1 binding activity of the CTB-cEDIII fusion protein in lyophilized transgenic rice callus was confirmed with both anti-CT and anti-dengue virus antibodies (Fig. 3a, b), indicating that plant-produced CTB-cEDIII fusion protein with biological activity was successfully expressed. GM1-ELISA was also used to evaluate the expression level of CTB-cEDIII fusion protein in lyophilized transgenic rice callus. The amount of CTB-cEDIII fusion protein was calculated via comparison with a known amount of bacterial CTB. The expression level of CTB-cEDIII protein was determined to be approximately 0.68 mg g−1 lyophilized rice callus using GM1-ELISA analysis with anti-CT antibody.
GM1-ELISA and quantification of CTB-cEDIII fusion proteins. The biological binding activity of CTB-cEDIII fusion proteins produced in transgenic rice callus to its GM1-ganglioside receptor was confirmed by GM1-ELISA with anti-CT (a) or anti-dengue virus antibodies (b). The production level of CTB-cEDIII fusion protein was measured by GM1-ELISA in lyophilized rice callus at day 7 after induction with sugar starvation (c)
Binding of CTB-scEDIII fusion protein to M cells in Peyer’s patches
Microfold (M) cells are specialized antigen sampling epithelial cells of the mucosal immune system. Therefore, the binding ability of CTB-cEDIII fusion proteins to Peyer’s patches was confirmed by a M cell binding assay in a mouse model. The M-cell-specific targeting ability of the functional CTB-cEDIII fusion protein expressed in transgenic rice callus was measured relative to UEA-1+WGA− M cells using fluorescence microscopy. Peyer’s patch tissue was incubated with transgenic rice callus protein extracts containing 50 μg of CTB-cEDIII fusion protein. Red represents UEA-1+WGA− M cells bound only with rhodamine-labeled UAE1 (red), purple represents enterocytes bound with rhodamine-labeled UAE1 (red) and Alexa Flour 350® conjugated-WGA (blue), yellow represents CTB-scEDIII fusion proteins (FITC-conjugated anti-mouse IgG, green) bound to M cells (red) and white represents CTB-scEDIII fusion protein (green) bound to enterocytes (purple). In both in vitro and in vivo assays, yellow and white coloring was detected, indicating that CTB-scEDIII fusion proteins were bound to the M cells and enterocytes in Peyer’s patches (Fig. 4). Green color was also detected together with yellow, indicating that CTB-cEDIII fusion protein was bound to M cells. CTB-cEDIII fusion proteins with green spots were widespread in the enterocytes of Peyer’s patches. There were no interactions between cEDIII alone or non-transgenic rice callus protein extracts with M cells or enterocytes in Peyer’s patches (Fig. 4).
Biological activity of plant-produced ligand fusion proteins in Peyer’s patches. The M cell-specific targeting abilities of the functional CTB-cEDIII fusion proteins produced in transgenic rice callus were measured relative to UEA-1+WGA− M cells in vitro (a) and in vivo (b) using fluorescence microscopy. Red color, rhodamine-labeled UEA-1 bound to M cells; green color, fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG bound with CTB-cEDIII fusion protein; yellow color, the combination of UEA-1 and cEDIII indicating the binding of CTB-cEDIII fusion proteins to M cells in the Peyer’s patches. (Color figure online)
Discussion
Many vaccine candidates against bacterial and viral pathogens have been produced in transgenic plant expression systems, as this method has the potential to meet the demand for safe and inexpensive vaccines (Loc et al. 2011; Martinez et al. 2011; Martinez-Gonzalez et al. 2011). However, low immune response and oral immune tolerance, which both are due to the low expression levels of target antigens in transgenic plants, are a barrier to plant-based oral vaccine development. The success of plant-based oral vaccines will depend on the efficiency of sampling antigens and to present them to the mucosal immune system in order to induce high immune responses against target antigens. Many efforts to improve the expression levels of target genes have used such approaches as strong or tissue-specific promoters, codon optimization of target genes, transcriptional or translational factors with 5′UTR sequences, protein targeting to subcellular locations, and fusion to a stable protein like the B subunit of the E. coli heat-labile enterotoxin (LT) or cholera toxin (CT) (Lau and Sun 2009).
The use of ligands is an alternative strategy to overcoming weak immune responses and immune tolerances. Ligands have the capacity to target fused antigens into mucosal immune systems in order to improve of antigen uptake and antigen presentation in antigen-presenting cells. The CTB was fused to an antigen and introduced into rice callus for this purpose. The CTB-cEDIII fusion protein was detected by Western blot analysis, and binding activity to its receptor, GM1-ganglioside, was confirmed by GM1-ELISA. The bands detected in Western blot analysis with anti-CT showed the same band position as detected with anti-dengue virus antibody, and GM1-ELISA showed that GM1–ganglioside-binding proteins interacted with both anti-CT and anti-dengue virus antibodies. These results indicate that plant-produced CTB-cEDIII fusion proteins contained both CTB and scEDIII domains.
M cells are specialized antigen-sampling epithelial cells in the mucosal immune system, and are easily accessible to microorganisms or migration molecules for tissue-specific consequences of lymphocyte priming in Peyer’s patches (Kim et al. 2010a; Takahashi et al. 2009). The binding ability of CTB-cEDIII fusion proteins to Peyer’s patches was confirmed by M cell binding assays in a mouse model. CTB-cEDIII fusion proteins (yellow, white, and green color, Fig. 4) were detected in the M cells and enterocytes of Peyer’s patches. This result indicates that the CTB-cEDIII fusion antigen protein, which is responsible for binding to GM1-gangliosides (present on all nucleated mammalian cells and abundant on intestinal epithelial cells), can be delivered into the M cells of the mucosal epithelium and successfully presented on the antigen-presenting cells of the mucosal immune system.
In Western blot analyses using anti-CT or anti-dengue virus antibody under boiled conditions, the CTB-cEDIII fusion proteins were detected as two bands of monomeric ligand fusion protein, which was similar to the banding pattern observed in previous studies investigating the expression of foreign proteins in transgenic plants (Kim et al. 2010c; Chebolu and Daniell 2009; Kim et al. 2009b; Arakawa et al. 1998). The two bands shown in Western blot analysis may account for the presence of processed or unprocessed RAmy3D signal peptides. Several bands, including two main bands (95 and 130 kDa), were detected by anti-CT and dengue virus antibodies in non-boiled conditions. The two main bands are assumed to be pentameric and tetrameric CTB-cEDIII fusion proteins because the monomer is slightly less than 26 kDa. Other faint bands were likely assembly of CTB-cEDIII fusion proteins.
The expression levels of CTB-cEDIII fusion proteins were measured in lyophilized transgenic rice callus. The expression level (0.68 mg/g) of CTB-cEDIII protein as measured by GM1-ELISA analysis, which assesses biological binding activity, was approximately 1.5 % of TSP in lyophilized transgenic rice callus. This expression level was considerably higher compared to previous experiments. EDIII and CTB-EDIII protein expressed in transgenic tobacco plants were found to be only 0.13–0.25 % of TSP based on Western blot analysis and 0.019 % of TSP based on GM1-ELISA (Kim et al. 2009a, 2010c). Plant-codon-optimized consensus EDIII protein produced in transgenic rice callus showed a high expression level of 0.45 mg g−1 lyophilized rice callus by densitometric analysis (Kim et al. 2012). EDIII expressed in plants using a tobacco mosaic virus vector system was detected at 0.28 % of TSP, and the purified EDIII protein induced the anti-dengue virus antibodies with neutralizing activity. Plant-produced EDIII protein did not elicit an immune response without an adjuvant, but a response was elicited with the use of an adjuvant (Saejung et al. 2007). CTB-cEDIII fusion proteins with high binding affinity to the mucosal inductive sites can be efficiently taken up into the mucosal immune system in order to improve the immune response against antigens and prevent infection with all dengue virus serotypes.
In this study, we developed a valuable plant-based oral vaccine against dengue virus infection. We constructed a plant expression plasmid to produce a fusion protein consisting of a plant-codon-optimized consensus EDIII and CTB under the control of the RAmy3D promoter expression system and transformed this plasmid into rice callus using a particle bombardment-mediated transformation method. The production of CTB-cEDIII fusion protein was confirmed by Western blot analysis and GM1-ELISA in transgenic rice callus. The CTB-cEDIII fusion protein expressed in transgenic rice callus showed binding activity to intestinal epithelial cells, including the M cells of gut-associated lymphoid tissues, by M cell binding assays in a mouse model. These results suggest the feasibility of the CTB-cEDIII fusion protein for improving the immune response by antigen targeting of the mucosal immune system.
Abbreviations
- CTB:
-
Cholera toxin B subunit
- LTB:
-
Enterotoxigenic E. coli enterotoxin B subunit
- scEDIII:
-
Synthetic consensus envelope protein domain III of dengue virus
- GM1-ELISA:
-
GM1-ganglioside enzyme-linked immunosorbent assay
- DHF:
-
Dengue hemorrhagic fever
- DSS:
-
Dengue shock syndrome
- ADE:
-
Antibody-dependent enhancement
- TSP:
-
Total soluble protein
- RAmy3D:
-
Rice amylase 3D
- UTRs:
-
Untranslated sequences
References
Arakawa T, Yu J, Chong DK, Hough J, Engen PC, Langridge WH (1998) A plant-based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nat Biotechnol 16:934–938
Batra G, Raut R, Dahiya S, Kamran N, Swaminathan S, Khanna N (2010) Pichia pastoris-expressed dengue virus type 2 envelope domain III elicits virus-neutralizing antibodies. J Virol Methods 167:10–16
Bendandi M, Marillonnet S, Kandzia R, Thieme F, Nickstadt A, Herz S, Frode R, Inoqes S, Lopez-Diaz de Cerio A, Soria E, Villanueva H, Vancanneyt G, McCormick A, Tuse D, Lenz J, Butler-Ransohoff JE, Klimyuk V, Gleba Y (2010) Rapid, high-yield production in plants of individualized idiotype vaccines for non-Hodgkin’s lymphoma. Ann Oncol 21:2420–2427
Block OK, Rodrigo WW, Quinn M, Jin X, Rose RC, Schlesinger JJ (2010) A tetravalent recombinant dengue domain III protein vaccine stimulates neutralizing and enhancing antibodies in mice. Vaccine 28:8085–8094
Boberg A, Gaunitz S, Brave A, Wahren B, Carlin N (2008) Enhancement of epitope-specific cellular immune responses by immunization with HIV-1 peptides genetically conjugated to the B-subunit of recombinant cholera toxin. Vaccine 26:5079–5082
Boyhan D, Daniell H (2011) Low-cost production of proinsulin in tobacco and lettuce chloroplasts for injectable or oral delivery of functional insulin and C-peptide. Plant Biotechnol J 9:585–598
Brandler S, Ruffie C, Najburg V, Frenkiel MP, Bedouelle H, Despres P, Tangy F (2010) Pediatric measles vaccine expressing a dengue tetravalent antigen elicits neutralizing antibodies against all four dengue viruses. Vaccine 28:6730–6739
Chebolu S, Daniell H (2009) Chloroplast-derived vaccine antigens and biopharmaceuticals: expression, folding, assembly and functionality. Curr Top Microbiol Immunol 332:33–54
Chen PW, Lu CA, Yu TS, Tseng TH, Wang CS, Yu SM (2002) Rice alpha-amylase transcriptional enhancers direct multiple mode regulation of promoters in transgenic rice. J Biol Chem 277:13641–13649
Chiang CY, Liu SJ, Tsai JP, Li YS, Chen MY, Liu HH, Chong P, Leng CH, Chen HW (2011) A novel single-dose dengue subunit vaccine induces memory immune responses. PLoS ONE 6:e23319
Etemad B, Batra G, Raut R, Dahiya S, Khanam S, Swaminathan S, Khanna N (2008) An envelope domain III-based chimeric antigen produced in Pichia pastoris elicits neutralizing antibodies against all four dengue virus serotypes. Am J Trop Med Hyg 79:353–363
Fonseca BA, Khoshnood K, Shope RE, Mason PW (1991) Flavivirus type-specific antigens produced from fusions of a portion of the E protein gene with the Escherichia coli trpE gene. Am J Trop Med Hyg 44:500–508
Gleba Y, Klimyuk V, Marillonnet S (2005) Magnifection-a new platform for expressing recombinant vaccines in plants. Vaccine 23:2042–2048
Gubler DJ (2002) Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol 10:100–103
Guzman MG, Hermida L, Bernardo L, Ramirez R, Guillen G (2010) Domain III of the envelope protein as a dengue vaccine target. Expert Rev Vaccin 9:137–147
Huang KJ, Yang YC, Lin YS, Huang JH, Liu HS, Yeh TM, Chen SH, Liu CC, Lei HY (2006) The dual-specific binding of dengue virus and target cells for the antibody-dependent enhancement of dengue virus infection. J Immunol 176:2825–2832
Huy NX, Yang MS, Kim TG (2011) Expression of a cholera toxin B subunit-neutralizing epitope of the porcine epidemic diarrhea virus fusion gene in transgenic lettuce (Lactuca sativa L.). Mol Biotechnol 48:201–209
Kim MY, Yang MS, Kim TG (2009a) Expression of dengue virus E glycoprotein domain III in non-nicotine transgenic tobacco plants. Biotechnol Bioprocess Eng 14:725–730
Kim TG, Huy NX, Kim MY, Jeong DK, Jang YS, Yang MS, Langridge WH, Lee JY (2009b) Immunogenicity of a cholera toxin B subunit Porphyromonas gingivalis fimbrial antigen fusion protein expressed in E. coli. Mol Biotechnol 41:157–164
Kim SH, Seo KW, Kim J, Lee KY, Jang YS (2010a) The M cell-targeting ligand promotes antigen delivery and induces antigen-specific immune responses in mucosal vaccination. J Immunol 185:5787–5793
Kim TG, Kim BG, Kim MY, Choi JK, Jung ES, Yang MS (2010b) Expression and immunogenicity of enterotoxigenic Escherichia coli heat-labile toxin B subunit in transgenic rice callus. Mol Biotechnol 44:14–21
Kim TG, Kim MY, Yang MS (2010c) Cholera toxin B subunit-domain III of dengue virus envelope glycoprotein E fusion protein production in transgenic plants. Protein Expr Purif 74:236–241
Kim MY, Kim TG, Yoo HS, Yang MS (2011) Expression and assembly of ApxIIA toxin of Actinobacillus pleuropneumoniae fused with the enterotoxigenic E. coli heat-labile toxin B subunit in transgenic tobacco. Plant Cell Tiss Organ Cult 105:375–382
Kim MY, Yang MS, Kim TG (2012) Expression of a consensus dengue virus envelope protein domain III in transgenic callus of rice. Plant Cell Tiss Organ Cult 109:509–515
Lau OS, Sun SS (2009) Plant seeds as bioreactors for recombinant protein production. Biotechnol Adv 27:1015–1022
Leng CH, Liu SJ, Tsai JP, Li YS, Chen MY, Liu HH, Lien SP, Yueh A, Hsiao KN, Lai LW, Liu FC, Chong P, Chen HW (2009) A novel dengue vaccine candidate that induces cross-neutralizing antibodies and memory immunity. Microbes Infect 11:288–295
Liljeqvist S, Stahl S, Andreoni C, Binz H, Uhlen M, Murby M (1997) Fusions to the cholera toxin B subunit: influence on pentamerization and GM1 binding. J Immunol Methods 210:125–135
Lipscombe M, Charles IG, Roberts M, Dougan G, Tite J, Fairweather NF (1991) Intranasal immunization using the B subunit of the Escherichia coli heat-labile toxin fused to an epitope of the Bordetella pertussis P.69 antigen. Mol Microbiol 5:1385–1392
Loc NH, Song NV, Tien NQD, Minh TT, Nga PTQ, Kim TG, Yang MS (2011) Expression of the Escherichia coli heat-labile enterotoxin B subunit in transgenic watercress (Nasturtium officinale L.). Plant Cell Tiss Organ Cult 105:39–45
Marillonnet S, Giritch A, Gils M, Kandzia R, Klimyuk V, Gleba Y (2004) In planta engineering of viral RNA replicons: efficient assembly by recombination of DNA modules delivered by Agrobacterium. Proc Natl Acad Sci USA 101:6852–6857
Martinez CA, Giulietti AM, Talou JR (2011) Expression of a KDEL-tagged dengue virus protein in cell suspension cultures of Nicotiana tabacum and Morinda citrifolia. Plant Cell Tiss Organ Cult 107:91–100
Martinez-Gonzalez L, Rosales-Mendoza S, Soria-Guerra RE, Moreno-Fierros L, Lopez-Revilla R, Korban SS, Guevara-Arauza JC, Alpuche-Solis AG (2011) Oral immunization with a lettuce-derived Escerichia coli heat-labile toxin B sububit induces neutralizing antibodies in mice. Plant Cell Tiss Organ Cult 107:441–449
Mason HS, Ball JM, Shi JJ, Jiang X, Estes MK, Arntzen CJ (1996) Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc Natl Acad Sci USA 93:5335–5340
Miller N (2010) Recent progress in dengue vaccine research and development. Curr Opin Mol Ther 12:31–38
Murrell S, Wu SC, Butler M (2011) Review of dengue virus and the development of a vaccine. Biotechnol Adv 29:239–247
Pelham HR (1990) The retention signal for soluble proteins of the endoplasmic reticulum. Trends Biochem Sci 15:483–486
Rosales-Mendoza S, Soria-Guerra RE, Moreno-Fierros L, Govea-Alonso DO, Herrera-Diaz A, Korban SS, Alpuche-Solis AG (2011) Immunogenicity of nuclear-encoded LTB: ST fusion protein from Escherichia coli expressed in tobacco plants. Plant Cell Rep 30:1145–1152
Saejung W, Fujiyama K, Takasaki T, Ito M, Hori K, Malasit P, Watanabe Y, Kurance I, Seki T (2007) Production of dengue 2 envelope domain III in plant using TMV-based vector system. Vaccine 25:6646–6654
Sanchez J, Holmgren J (2008) Cholera toxin structure, gene regulation and pathophysiological and immunological aspects. Cell Mol Life Sci 65:1347–1360
Sanchez AE, Aquino G, Ostoa-Saloma P, Laclette JP, Rocha-Zavaleta L (2004) Cholera toxin B-subunit gene enhances mucosal immunoglobulin A, Th1-type, and CD8 + cytotoxic responses when coadministered intradermally with a DNA vaccine. Clin Diagn Lab Immunol 11:711–719
Schodel F, Enders G, Jung MC, Will H (1990) Recognition of a hepatitis-B virus nucleocapsid T-cell epitope expressed as a fusion protein with the subunit-B of Escherichia coli heat labile enterotoxin in attenuated salmonellae. Vaccine 8:569–572
Shin YJ, Hong SY, Kwon TH, Jang YS, Yang MS (2003) High level of expression of recombinant human granulocyte-macrophage colony stimulating factor in transgenic rice cell suspension culture. Biotechnol Bioeng 82:778–783
Shin MK, Jung MH, Lee WJ, Choi PS, Jang YS, Yoo HS (2011) Generation of transgenic corn-derived Actinobacillus pleuromoniae ApxIIA fused with the cholera toxin B subunit as a vaccine candidate. J Vet Sci 12:401–403
Siddiqui FJ, Haider SR, Bhutta ZA (2009) Endemic dengue fever: a seldom recognized hazard for Pakistani children. J Infect Dev Ctries 3:306–312
Streatfield SJ (2006) Mucosal immunization using recombinant plant-based oral vaccines. Methods 38:150–157
Swaminathan S, Batra G, Khanna N (2010) Dengue vaccines: state of the art. Expert Opin Ther Pat 20:819–835
Takahashi I, Nochi T, Yuki Y, Kiyono H (2009) New horizon of mucosal immunity and vaccines. Curr Opin Immunol 21:352–358
Yasuda H, Hayashi Y, Jomori T, Takaiwa F (2006) The correlation between expression and localization of a foregin gene product in rice endosperm. Plant Cell Physiol 47:756–763
Youm JW, Jeon JH, Kim H, Min SR, Kim MS, Joung H, Jeong WJ, Kim HS (2010) High-level expression of a human beta-site APP cleaving enzyme in transgenic tobacco chloroplasts and its immunogenicity in mice. Transgenic Res 19:1099–1108
Yuki Y, Tokuhara D, Nochi T, Yasuda H, Mejima M, Kurokawa S, Takahashi Y, Kataoka N, Nakanishi U, Hagiwara Y, Fujihashi K, Takaiwa F, Kiyono H (2009) Oral MucoRice expressing double-mutant cholera toxin A and B subunits induces toxin-specific neutralising immunity. Vaccine 27:5982–5988
Acknowledgments
This study was supported by the Bio-industry Technology Development Program, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, MY., Chung, ND., Yang, MS. et al. Expression of a cholera toxin B subunit and consensus dengue virus envelope protein domain III fusion gene in transgenic rice callus. Plant Cell Tiss Organ Cult 112, 311–320 (2013). https://doi.org/10.1007/s11240-012-0238-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-012-0238-2