Abstract
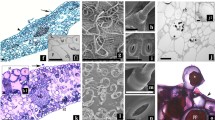
The anatomy of normal and hyperhydric shoots (leaves and stems) of in vitro Handroanthus impetiginosus was compared using light microscopy, scanning electron microscopy, and transmission electron microscopy. In contrast to normal shoots, hyperhydric shoots presented numerous anatomical abnormalities at the proliferation stage. Disorganized cortex, epidermal holes, epidermal discontinuity, collapsed cells, and other structural characteristics were observed in hyperhydric shoots. So, by using anatomical analysis of in vitro H. impetiginosus shoots at the proliferation stage, we can predict which plants will survive the rhizogenesis and acclimatization stages.





Similar content being viewed by others
References
Amaral da Silva EA, Davide AC, Rocha Faria JM, Bandeira de Melo DL, Barbosa de Abreu G (2004) Germination studies on Tabebuia impetiginosa Mart. seeds. Cerne 10(1):1–9
Apóstolo NM, Llorente B (2000) Anatomy of normal and hyperhydric leaves and shoots of in vitro grown Simmondsia chinesis (Link) Schn. In Vitro Cell Dev Biol Plant 36(4):243–249
Apóstolo NM, Bryant ME, Jausoro V, Llorente BE (2005) Optimización del medio de cultivo para la multiplicación in vitro de lapacho Tabebuia impetiginosa (Mart. ex. DC) Standl. (Bignoniaceae). Poster. Bairesbiotec, REDBio 2005. Capital Federal, 7 al 10 de junio de 2005. Libro de resúmenes: 98
Avato P, Fortunato IM, Ruta C, D’Elia R (2005) Glandular hairs and essential oils in micropropagated plants of Salvia officinalis L. Plant Sci 169:29–36
Bandyopadhyay T, Gangopadhyay G, Poddar R, Mukherjee KK (2004) Trichomes: their diversity, distribution and density in acclimatization of teak (Tectona grandis L.) plants grown in vitro. Plant Cell Tiss Organ Cult 78:113–121
Carvalho PER (1994) Espécies Florestais brasileiras: recomendações silviculturais, potencialidades e uso da madeira. EMBRAPA-CNPF, Colombo, p 640
D’Ambrogio de Argüeso A (1986) Manual de Técnicas en Histología Vegetal. Editorial Hemisferio Sur, Buenos Aires, Argentina
Debergh P, Aitken-Christie J, Cohen D, Grout B, von Arnold S, Zimmerman R, Ziv M (1992) Reconsideration of the term “vitrification” as used in micropropagation. Plant Cell Tiss Org Cult 30:135–140
Dousseau S, Alves de Alvarenga A, Mauro de Castro E, Pereira Soares R, Bucsan Emrich E, Amaral de Melo L (2008) Anatomia foliar de Tabebuia serratifolia (Vahl) Nich. (Bignoniaceae) propagadas in vitro, in vivo e durante a aclimatização. Ciênc Agrotec Lavras 32(6):1694–1700
Fontes MA, Otoni WC, Carolino SMB, Brommonschenkel SH, Fontes EPB, Fári M, Louro RP (1999) Hyperhydricity in pepper plants regenerated in vitro: involvement of BiP (binding protein) and ultrastructural aspects. Plant Cell Rep 19:81–87
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gribble K, Sarafis V, Nailon J, Holford P, Uwins P (1996) Environmental scanning electron microscopy of the surface of normal and vitrified leaves of Gypsophila paniculata (babies breath) cultured in vitro. Plant Cell Rep 15:771–776
Gribble K, Sarafis V, Conroy J (2003) Vitrified plants: towards an understanding of their nature. Phytomorphology 53(1):1–10
Grose SO, Olmstead RG (2007a) Evolution of a charismatic neotropical clade: molecular phylogeny of Tabebuia s. l., Crescentieae, and allied genera (Bignoniaceae). Syst Bot 32(3):650–659
Grose SO, Olmstead RG (2007b) Taxonomic revisions in the polyphyletic genus Tabebuia s. l. (Bignoniaceae). Syst Bot 32(3):660–670
Justiniano MJ, Fredericksen TS, Nash D (2000) Ecología y silvicultura de especies menos conocidas—Tajibos o Lapachos (Tabebuia spp.). Editora El Pais, Santa Cruz, Bolivia
Koyama J, Morita I, Tagahara K, Hirai K (2000) Cyclopentene dialdehydes from Tabebuia impetiginosa. Phytochemistry, Oxford 53(8):869–872
Lahitte HB, Hurrell JA, Valla JJ, Jankowski L, Bazzano D, Hernández AJ (1999) Arboles Urbanos. Biota Rioplatense IV. Ediciones LOLA, Buenos Aires, Argentina
Leonardi D, Di Sapio O, Gattuso M, Gattuso S (2002) Caracteres morfoanatómicos de corteza y hoja de Tabebuia impetiginosa (Mart. ex DC.) Standl. y Tabebuia heptaphylla (Vell.) Tol. Bol Soc Argent Bot 37(1–2):51–61
Lorenzi H (1992) Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas nativas do Brasil. Editorial Plantarum, Nova Odessa, p 352
Louro RP, Dos Santos AV, Machado RD (1999) Ultrastructure of Eucalyptus grandis × Eucalyptus urophylla. 1. Shoots cultivated in vitro in multiplication and elongation-rooting media. Int J Plant Sci 160(2):217–227
Lozano EC, Zapater MA (2008) Delimitación y estatus de Handroanthus heptaphyllus y H. impetiginosus (Bignoniaceae, Tecomeae). Darviniana 46(2):304–317
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Olmos E, Hellin E (1998) Ultrastructural differences of hyperhydric and normal leaves from regenerated carnation plants. Sci Hortic 75:91–101
Picoli EAT, Otoni WC, Figueira ML, Carolino SMB, Almeida RS, Silva EAM, Carvalho CR, Fontes EPB (2001) Hyperhydricity in in vitro eggplant regenerated plants: structural characteristics and involvement of BiP (binding protein). Plant Sci 160:857–868
Ponessa GI, Parrado MF, Guantay ME (2005) Caracteres morfoanatomicos de hoja, corteza y leño de Tabebuia impetiginosa (Mart. ex DC.) Standl. (Bignoniaceae). Rojasiana 6(2):131–140
Portas AM, Medrano N, Díaz M, Leiva N, Gianfrancisco S, Figueroa de Orell MI (2008) El cultivo sin suelo en la producción de raíces en estacas leñosas de Tabebuia (Root production of Tabebuia grafting cultured without soil). IV Argentine Congress of Flowerculture and Ornamental plants. Corrientes
Willmer C, Fricker M (1996) Stomata, 2nd edn. Chapman & Hall, London, UK
Zapater MA, Califano LM, del Castillo EM, Quiroga MA, Lozano EC (2009) Las especies nativas y exóticas de Tabebuia y Handroanthus (Tecomeae, Bignoniaceae) en Argentina. Darwiniana 47(1):185–220
Ziv M (1991) Vitrification: morphological and physiological disorders of in vitro plants. In: Debergh PC, Zimmerman RH (eds) Micropropagation. Technology and application. Kluwer Academic Publishers, The Netherlands, pp 45–69
Ziv M, Ariel T (1992) On the relation between vitrification and stomatal cell wall deformity in carnation leaves in vitro. Acta Hortic 314:121–129
Ziv M, Chen J (2008) The anatomy and morphology of tissue cultured plants. In: George EF, Hall MA, de Klerk G-J (eds) Plant propagation by tissue culture, 3rd edn. Springer, The Netherlands, pp 465–479
Acknowledgments
This research was supported by a grant from the Department of Basic Sciences, National University of Luján.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jausoro, V., Llorente, B.E. & Apóstolo, N.M. Structural differences between hyperhydric and normal in vitro shoots of Handroanthus impetiginosus (Mart. ex DC) Mattos (Bignoniaceae). Plant Cell Tiss Organ Cult 101, 183–191 (2010). https://doi.org/10.1007/s11240-010-9675-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9675-y