Abstract
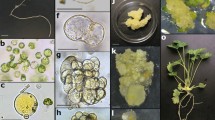
Protoplasts were isolated from leaves of in vitro-grown shoot cultures of different Petunia and Calibrachoa genotypes by enzyme digestion with 0.6% macerozyme R-10 and 2.0% cellulase R-10. Shoot regeneration was achieved in five out of nine Calibrachoa and three out of four Petunia genotypes. Protoplast yield and frequency of shoot regeneration varied among experiments and genotypes. Among all regenerants, few morphological changes, such as chlorophyll deficiency, variegated leaves, and polyploidization, were observed.




Similar content being viewed by others
Abbreviations
- BA:
-
6-Benzyladenine
- FW:
-
Fresh weight
- IAA:
-
Indole-3-acetic acid
- MS:
-
Murashige and Skoog (1962)
- NAA:
-
1-Naphthaleneacetic acid
- TDZ:
-
Thidiazuron
References
Bajaj YPS, Davey MR (1974) The isolation and ultrastructure of pollen protoplasts. In: Linskens HF (ed) Fertilization of higher plants. North Holland, Amsterdam, pp 73–80
Bhojwani SS, Radzan MK (1996) Plant tissue culture: theory and practice. Elsevier, Amsterdam
Binding H (1974) Regeneration von haploiden und diploiden Pflanzen aus Protoplasten von Petunia hybrida L. Zeitschrift f Pflanzenphysiol 74:327–356
Creemers-Molenaar J, Loeffen JPM, van der Valk P (1988) The effect of 2, 4-dichlorophenoxyacetic acid and donor plant environment on plant regeneration from immature inflorescence-derived callus of Lolium perenne L. and Lolium multiflorum L. Plant Sci 57:165–172
De Laat AMM, Göhde W, Vogelzang MJDC (1987) Determination of ploidy of single plants and plant populations by flow cytometry. Plant Breed 99:303–309
Feng GH, Ouyang RG (1988) The effect of KNO3 concentration in callus induction medium for wheat anther culture. Plant Cell Tissue Organ Cult 12:3–12
Ford-Logan J, Sink KC (1988) Plantlet regeneration from protoplasts of Petunia alpicola. HortSci 23(2):393–395
Frearson EM, Power JB, Cocking EC (1973) The isolation, culture and regeneration of Petunia leaf protoplasts. Dev Biol 33:130–137
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension culture of soybean root cells. Exp Cell Res 50:151–158
Golds TJ, Babczinsky J, Rauscher G, Koop HU (1992) Computer controlled tracking of single cell development in Nicotiana tabacum L. and Hordeum vulgare L. protoplasts embedded in agarose/alginate films. J Plant Physiol 140:582–587
Hayward C, Power JB (1975) Plant production from leaf protoplasts of Petunia parodii. Plant Sci Lett 4:407–410
Izhar S, Power JB (1977) Genetical studies with Petunia leaf protoplasts 1. Genetic variation to specific growth hormones and possible genetic control on stages of protoplast development in culture. Plant Sci Lett 8:375–383
Jussieu AL (1803) Sur le Petunia, genre nouveau de la famille des plants solanees. Ann Mus Hist Nat 2:214–216 In Tsukamoto T, Ando T, Watanabe H, Kokubun H, Hashimoto G, Sakazaki U, Suarez E, Marchesi, E, Oyama K, Kao T-H (2002) Differentiation in the status of self-incompatibility among Calibrachoa species (Solanaceae). J Plant Res 115:185-193
Kao KN, Michayluk MR (1975) Nutritional requirements for growth of Vicia hajastana cells and protoplasts at a very low population density in liquid media. Planta 126:105–110
Kao KN, Gamborg OL, Miller RA, Keller WA (1971) Cell divisions in cells regenerated from protoplasts of soybean and Haplopappus gracilis. Nat New Biol 232:124
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–479
Nagata T, Takebe I (1971) Plating of isolated tobacco mesophyll protoplasts on agar medium. Planta 99:12–20
Oh MH, Kim SG (1994) Plant regeneration from petal protoplast culture of Petunia hybrida. Plant Cell Tissue Organ Cult 36:275–283
Oh MH, Choi DW, Kwon YM, Kim SG (1995) An assessment of cytological stability in protoplast cultures of tetraploid Petunia hybrida. Plant Cell Tissue Organ Cult 41:243–248
Ouyang JW, Zhou SM, Jia SE (1983) The response of anther culture to culture temperature in Triticum aestivum. Theor Appl Genet 66:101–109
Pati PK, Sharma M, Ahuja PS (2005) Extra thin alginate film: an efficient technique for protoplast culture. Protoplasma 226:217–221
Potrykus I (1971) Fusion von Protoplasten mit gut sichtbaren Kernen. Naturwissenschaften 58:328
Power JB, Berry SF, Chapman JV, Cocking EC (1979) Somatic hybrids between unilateral cross—incompatible Petunia species. Theor Appl Genet 55:97–99
Power JB, Berry SF, Chapman JV, Cocking EC (1980) Somatic hybridization of sexually incompatible Petunias: Petunia parodii, Petunia parviflora. Theor Appl Genet 57:1–4
Raveh D, Huberman E, Galun E (1973) In vitro culture of tobacco protoplasts: use of feeder techniques to support division of cells plated at low densities. In vitro 9:216–222
Renaudin JP, Tournaire C, Brillat M, De La Serve TB (1990) Sequential hormone requirement for growth and organogenesis of Petunia hybrida protoplasts-derived calli. Plant Sci 71:239–250
Shillito RD, Paszkowski J, Potrykus I (1983) Agarose plating and a bead type culture technique enable and stimulate development of protoplast-derived colonies in a number of plant species. Plant Cell Rep 2:244–247
Taguchi T, Sakamoto K, Terada M (1993) Fertile somatic hybrids between Petunia hybrida and a wild fertile species Petunia variabilis. Theor Appl Genet 87:75–80
Tricoli DM, Hein MB, Carnes MG (1986) Culture of soybean mesophyll protoplasts in alginate beads. Plant Cell Rep 5:334–337
Ulrich I, Ulrich W (1991) High-resolution flow cytometry of nuclear DNA in higher plants. Protoplasma 165:212–215
Widholm JM (1972) The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol 47:189–194
Wijsman HJW (1990) On the inter-relationships of certain species of Petunia VI. New names for the species of Calibrachoa formerly included into Petunia (Solanaceae). Acta Bot 39(1):101–102
Winkelmann T, Grunewaldt J (1992) Plant regeneration from protoplasts of Saintpaulia ionantha (H. Wendl). Gartenbauwissenschaften 57:284–287
Winkelmann T, Grunewaldt J (1995) Analysis of protoplast-derived plants of Saintpaulia ionantha H. Wendl. Plant Breed 114:346–350
Winkelmann T, Specht J, Serek M (2006) Efficient plant regeneration from protoplasts isolated from embryogenic suspension cultures of Cyclamen persicum Mill. Plant Tiss Org Cult 86:337–347
Acknowledgments
This project was funded by the Federal Ministry of Economy and Technology (BMWi). The authors thank Dr. Andrea Dohm, breeding director Selecta Klemm GmbH, for excellent cooperation during this study, and Professor Errol Hewett (Massey University, Palmerston North, New Zealand) for linguistic editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meyer, L., Serek, M. & Winkelmann, T. Protoplast isolation and plant regeneration of different genotypes of Petunia and Calibrachoa . Plant Cell Tiss Organ Cult 99, 27–34 (2009). https://doi.org/10.1007/s11240-009-9572-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-009-9572-4